cGAS STING Pathway activation by DNA Plasmid Contamination, SPIKE, and LPS in modRNA "vaccines": AIDP, Myocarditis, Stroke, Aortic Dissection, and More: Overview, and Biopsy Methods for Detection.
SUPER cGAS STING SUBSTACK: Detection methods for scientists/pathologists towards the end
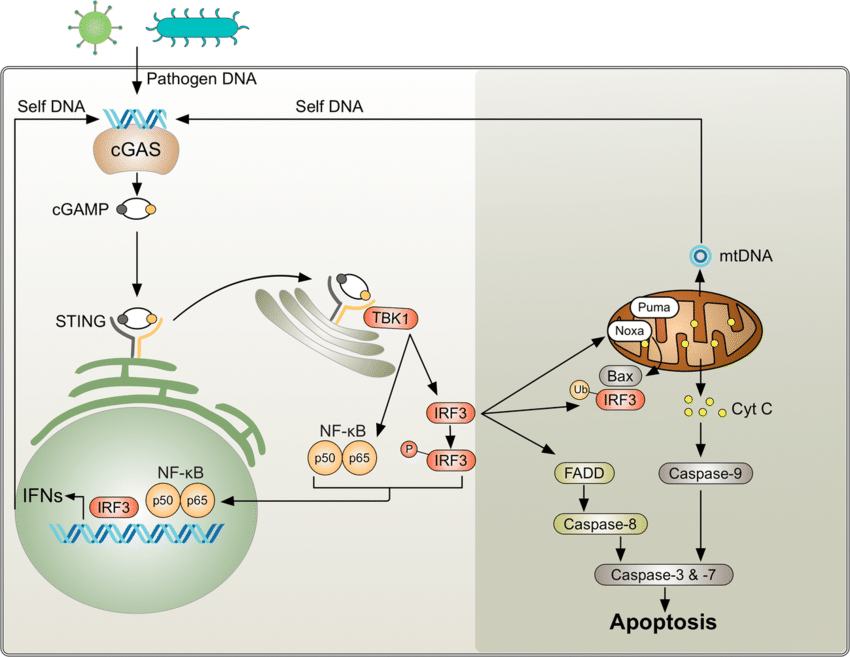
cGAS STING PATHWAY
The cGAS-STING pathway is a critical component of the innate immune system. It serves as sensor for cytosolic DNA and orchestrating the cellular response to various microbial infections, cellular stress, and DNA damage.
You could think of it like a smoke detector sensing smoke, and then activating an alarm system, that responds to danger, but worse.
Basic Overview:
I. Components of the Pathway:
cGAS (cyclic GMP-AMP synthase):
cGAS is a cytosolic DNA sensor that recognizes double-stranded DNA (dsDNA) derived from pathogens, damaged cells, or cellular debris.
Upon DNA binding, cGAS catalyzes the synthesis of cyclic GMP-AMP (cGAMP) from ATP and GTP.
STING (Stimulator of Interferon Genes):
STING is an endoplasmic reticulum (ER)-resident protein that serves as a signaling adaptor downstream of cGAS.
Upon binding to cGAMP or other cyclic dinucleotides (CDNs), STING undergoes conformational changes and translocates from the ER to perinuclear puncta, where it recruits downstream signaling effectors.
II. Activation Mechanisms:
Activation by Exogenous DNA:
Recognition of Pathogen DNA: cGAS detects cytosolic DNA derived from bacteria, viruses, or other pathogens.
Pathogen DNA may be released during infection, replication, or cell lysis.
DNA that is exogenous DOES include what is called ODN—the pieces of plasmid DNA that are in the current modRNA “vaccines” will also activate this pathway.
Binding to cGAS:
Double-stranded DNA (dsDNA) binds to the catalytic domain of cGAS, inducing a conformational change that activates its enzymatic activity.
cGAS then catalyzes the formation of 2'3'-cGAMP, a cyclic dinucleotide (CDN) second messenger.
Activation by Viral Infections:
Viral Genome Sensing:
During viral infections, viral nucleic acids, such as DNA or RNA, can trigger the cGAS-STING pathway.
DNA viruses, retroviruses, and RNA viruses with DNA intermediates are detected by cGAS, while RNA viruses may activate STING indirectly through mechanisms involving RNA-DNA hybrids or mitochondrial antiviral signaling proteins (MAVS).
The spike protein has been found in research literature to activate this pathway.
Activation by Endotoxin (LPS):
TLR4-Mediated Induction: Endotoxin, or lipopolysaccharide (LPS), activates Toll-like receptor 4 (TLR4) on the cell surface of immune cells like macrophages and dendritic cells.
TLR4 signaling induces the production of type I interferons (IFNs) and pro-inflammatory cytokines, which can indirectly activate the cGAS-STING pathway through cellular stress and DNA damage responses.
III. Signaling Cascade:
STING Activation:
Upon binding to cGAMP or CDNs, STING undergoes a conformational change, leading to its oligomerization and recruitment of downstream signaling effectors.
TBK1 Activation:
STING recruits TANK-binding kinase 1 (TBK1) to perinuclear puncta, where TBK1 phosphorylates STING and other substrates, including IRF3 (interferon regulatory factor 3).
IFN Induction:
Phosphorylated IRF3 translocates to the nucleus and induces the expression of type I interferons (IFN-α and IFN-β), which act in an autocrine and paracrine manner to stimulate antiviral and inflammatory responses.
IV. Cellular Distribution:
Ubiquitous Expression:
cGAS is expressed in most cell types, including immune cells, epithelial cells, and fibroblasts, whereas STING is predominantly expressed in immune cells but can also be found in non-immune cells.
Subcellular Localization:
cGAS is primarily cytosolic, whereas STING localizes to the endoplasmic reticulum (ER) in resting cells and redistributes to perinuclear puncta upon activation.
Activation Sites:
Infection Sites: The cGAS-STING pathway is activated in response to microbial infections at the site of infection, where pathogens release DNA or RNA into the cytosol.
Inflamed Tissues: Inflammatory conditions, such as autoimmune diseases, cancer, or tissue injury, can lead to the release of self-DNA or DAMPs (damage-associated molecular patterns), activating the cGAS-STING pathway in infiltrating immune cells or stromal cells.
V. Physiological and Pathological Functions:
Antimicrobial Defense:
Viral Immunity:
Activation of the cGAS-STING pathway induces the expression of type I interferons and other antiviral effectors, leading to the inhibition of viral replication, clearance of infected cells, and activation of adaptive immune responses.
Bacterial Immunity:
The cGAS-STING pathway contributes to host defense against intracellular bacterial pathogens by promoting the production of pro-inflammatory cytokines, antimicrobial peptides, and reactive oxygen species (ROS).
Autoimmunity and Inflammation:
Autoimmune Diseases:
Dysregulation of the cGAS-STING pathway has been implicated in various autoimmune diseases, including systemic lupus erythematosus (SLE), Aicardi-Goutières syndrome (AGS), and type I interferonopathies, where aberrant activation of the pathway leads to the production of autoantibodies, tissue inflammation, and organ damage.
Chronic Inflammation:
Prolonged activation of the cGAS-STING pathway can contribute to chronic inflammation, tissue fibrosis, and the pathogenesis of inflammatory disorders, such as inflammatory bowel disease (IBD), rheumatoid arthritis, and neurodegenerative diseases.
VI. How the pathway hits you “twice”:
In the case of exogenous DNA interacting, such as pieces of DNA plasmid inside an LNP:
(simplest terms—the exogenous pieces of DNA plasmid are recognized by the cGAS STING pathway, which then is activated, and signals the immune system to send “help” to the area, which inflames the tissues and cells, causing the cells to release “their own” DNA (human DNA), which then causes the pathway and immune system to recognize “self” DNA in places it does not belong, which causes the immune system to “attack” the area again. this can be sustained through a positive feedback loop, even when the foreign DNA is no longer there. This will be explained in MUCH detail.
Details of cGAS STING pathway Activation by Exogenous pieces of Plasmid DNA:
Activation by Exogenous DNA:
The cGAS-STING pathway can be activated when cytoplasmic DNA is detected, including exogenous DNA from pathogens or damaged self-DNA released from cells.
cGAS (cyclic GMP-AMP synthase) is a cytosolic DNA sensor that recognizes double-stranded DNA (dsDNA) and catalyzes the production of cyclic GMP-AMP (cGAMP).
Immune Activation:
Upon activation, cGAS generates the second messenger cGAMP, which binds to and activates the adaptor protein STING (Stimulator of Interferon Genes).
Activated STING triggers downstream signaling cascades, leading to the production of type I interferons (IFNs) and other inflammatory cytokines.
Inflammatory Response:
Type I interferons and inflammatory cytokines induce an inflammatory response, recruiting immune cells such as macrophages, dendritic cells, and T cells to the site of infection or tissue damage.
These immune cells eliminate pathogens and clear cellular debris, but excessive inflammation contributes to tissue damage and pathology.
Release of Self-DNA:
Inflammatory processes can lead to cell death or damage, resulting in the release of self-DNA from damaged or dying cells.
This self-DNA can further activate the cGAS-STING pathway in neighboring cells or immune cells, perpetuating the immune response.
Positive Feedback Loop:
The release of self-DNA and continued activation of the cGAS-STING pathway create a positive feedback loop, amplifying the immune response and sustaining inflammation.
This amplification mechanism can contribute to chronic inflammation and autoimmune diseases if dysregulated, including MS, AIDP, Sjögren's Syndrome, Myocarditis, and more.
Immune Attack on Self-DNA:
The immune system may recognize self-DNA as foreign or aberrant, leading to an autoimmune response against host tissues. Immune cells, particularly T cells and autoantibodies, may target self-DNA and contribute to tissue damage in autoimmune diseases such as lupus, arthritis, or inflammatory bowel disease.
(check out the current study on children suffering from IBS, and the detection of foreign DNA activating the cGAS STING pathway which is in process, right as I type:
https://threadreaderapp.com/thread/1740253978988867633.html
Exploration of the Activity of DNA Located Outside of Cellular Nucleus to Amplify Inflammation in Inflammatory Bowel Disease in Children Through Biological Pathway Cyclic GMP-AMP Synthase (cGAS) - Stimulator of Interferon Genes (STING) (ROXANE)
VII: The FEEDBACK LOOP: HOW the cGAS STING PATHWAY IS MAINTAINED IN THE “ON” POSITION—CONSTANT INFLAMMATION, AUTOIMMUNE ATTACK, AND WORSENING OF CONDITIONS EVEN IF THERE IS NO LONGER A PRESENCE OF PLASMID DNA, SPIKE PROTEIN, OR LPS:
Positive Feedback Loops:
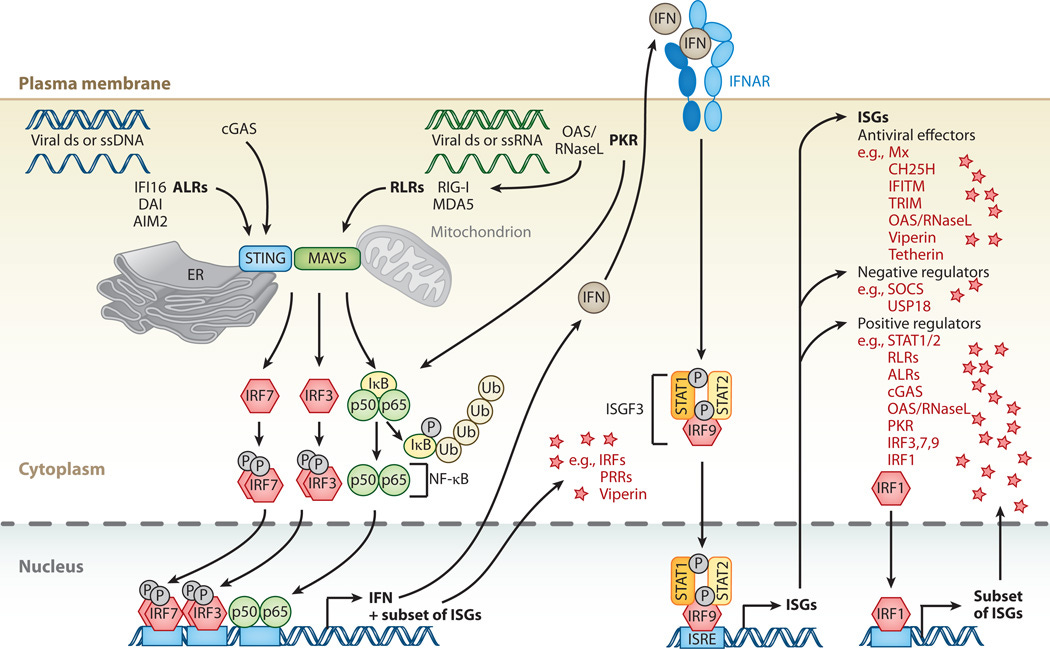
Upon activation, the cGAS-STING pathway triggers the production of type I interferons (IFNs) and other inflammatory cytokines, which in turn promote the expression of interferon-stimulated genes (ISGs).
Some ISGs encode proteins that directly amplify the cGAS-STING signaling pathway or modulate its activity, creating a positive feedback loop that reinforces immune responses.
These proteins may include factors involved in signal transduction, transcriptional regulation, or post-translational modifications.
Inflammatory Signaling Cascades:
In addition to type I IFNs, other pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) can activate downstream signaling pathways, including NF-κB and MAPK pathways.
These signaling cascades further enhance the expression of inflammatory mediators, perpetuating inflammation and immune cell activation.
Chronic Tissue Damage and Inflammation:
Prolonged activation of the cGAS-STING pathway and sustained immune responses can lead to chronic tissue damage and inflammation.
Cellular stress, DNA damage, and mitochondrial dysfunction may exacerbate cGAS-STING activation, creating a cycle of tissue injury and immune activation.
Damaged tissues release danger-associated molecular patterns (DAMPs), including self-DNA, ATP, and HMGB1, which further stimulate innate immune responses and maintain inflammation.
Autoimmune Responses:
In autoimmune diseases, the cGAS-STING pathway may become dysregulated, leading to the recognition of self-DNA as foreign and the generation of autoantibodies against self-antigens. Autoantibodies, particularly those targeting nuclear antigens, can form immune complexes that activate complement cascades and recruit immune cells, perpetuating tissue inflammation and injury.
Epigenetic Regulation:
Epigenetic modifications, including DNA methylation, histone modifications, and non-coding RNA regulation, can modulate the activity of genes involved in the cGAS-STING pathway and immune responses. Epigenetic changes induced by chronic inflammation or cellular stress can contribute to the persistence of immune activation and the development of autoimmune phenotypes.
VIII. Super deep dive on the feedback loops:
Positive Feedback Loops:
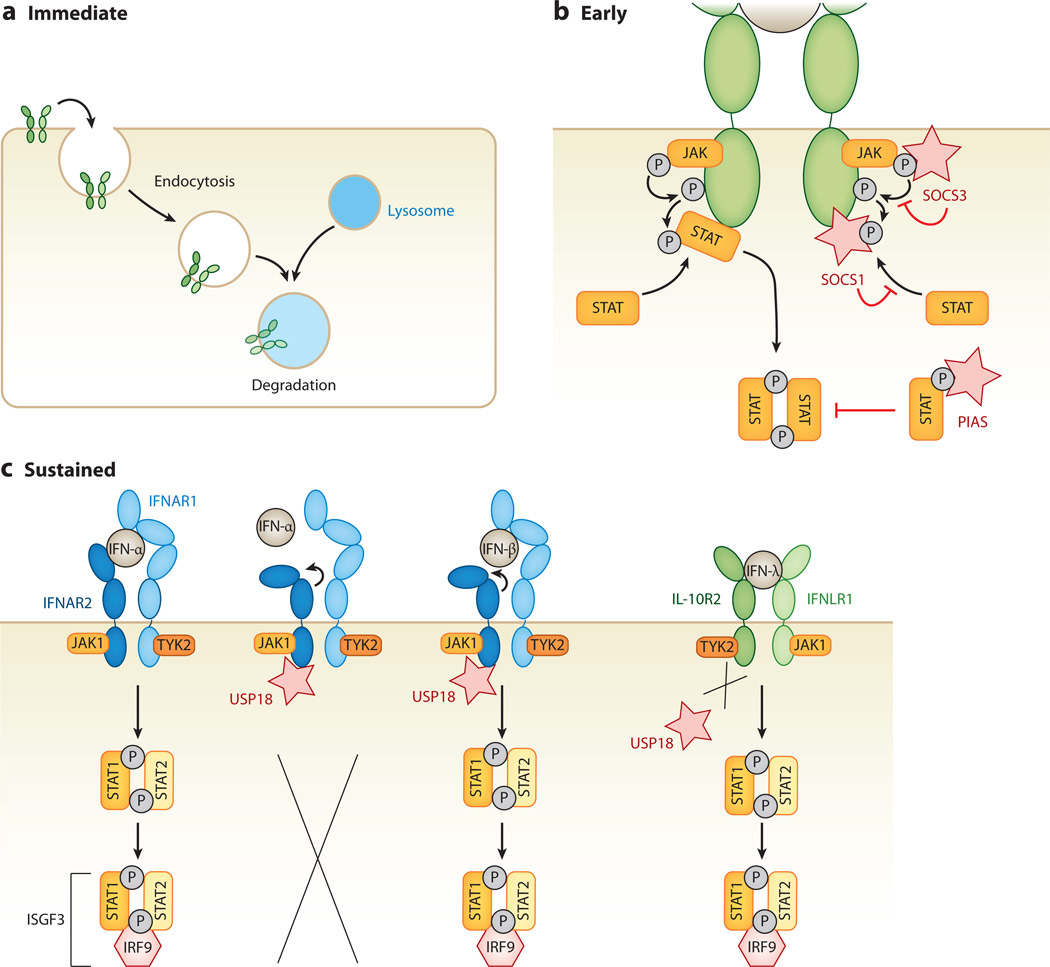
Interferon-Stimulated Genes (ISGs):
Upon activation by type I interferons (IFNs), ISGs are upregulated, many of which encode proteins involved in amplifying the cGAS-STING signaling pathway.
For example, IFN-induced proteins such as IFIT1, IFIT3, and MX1 can directly interact with components of the pathway to enhance signaling or promote downstream effector functions.
Amplification of Signaling Pathways:
Some ISGs may modulate the activity of key signaling molecules involved in the cGAS-STING pathway, such as TBK1 (TANK-binding kinase 1) and IRF3 (interferon regulatory factor 3), leading to the amplification of downstream signaling events and the sustained production of inflammatory mediators.
Inflammatory Signaling Cascades:
NF-κB Pathway:
Activation of NF-κB signaling by inflammatory cytokines such as TNF-α and IL-1β can synergize with the cGAS-STING pathway to enhance the expression of pro-inflammatory genes.
NF-κB target genes include cytokines, chemokines, and adhesion molecules that recruit immune cells to sites of inflammation and promote tissue damage.
MAPK Pathway:
Mitogen-activated protein kinase (MAPK) signaling pathways, including the ERK, JNK, and p38 MAPK pathways, can be activated by inflammatory stimuli and contribute to the regulation of gene expression, cell proliferation, and immune responses.
MAPK activation downstream of cGAS-STING signaling may further amplify inflammatory signaling cascades and immune cell activation.
Chronic Tissue Damage and Inflammation:
Cellular Stress and DNA Damage:
Persistent activation of the cGAS-STING pathway and sustained immune responses can lead to cellular stress, DNA damage, and mitochondrial dysfunction.
Accumulation of damaged cellular components and reactive oxygen species (ROS) contributes to tissue injury and inflammation, perpetuating immune activation and exacerbating tissue damage.
Danger-Associated Molecular Patterns (DAMPs):
Damaged or stressed cells release DAMPs, including self-DNA, ATP, and HMGB1, which serve as danger signals to activate innate immune responses.
DAMPs can engage pattern recognition receptors (PRRs) on immune cells, triggering inflammatory signaling cascades and amplifying immune activation.
Autoimmune Responses:
Loss of Self-Tolerance:
Dysregulated activation of the cGAS-STING pathway and prolonged exposure to self-DNA can lead to the breakdown of immune tolerance and the generation of autoreactive immune responses.
Autoantibodies targeting self-antigens, such as nuclear antigens in systemic lupus erythematosus (SLE), form immune complexes that drive tissue inflammation and contribute to autoimmune pathology.
Epigenetic Regulation:
DNA Methylation and Histone Modifications:
Epigenetic modifications can regulate the expression of genes involved in the cGAS-STING pathway and immune responses.
Changes in DNA methylation patterns and histone modifications may alter the accessibility of gene promoters, enhancers, and regulatory elements, influencing the transcriptional activity of key immune genes.
Non-Coding RNA Regulation:
MicroRNAs (miRNAs) and long non-coding RNAs (lncRNAs) can modulate the expression of genes in the cGAS-STING pathway and immune signaling networks.
Dysregulation of miRNA or lncRNA expression may contribute to aberrant immune activation and autoimmune diseases by affecting the stability of mRNA transcripts or the activity of signaling proteins.
IX. How cGAS STING (and also GAMP) activation can HURT YOU:
Type I Interferon (IFN) Production:
Upon activation by cytosolic DNA, cGAS catalyzes the production of cyclic GMP-AMP (cGAMP).
cGAMP serves as a second messenger that binds to and activates the Stimulator of Interferon Genes (STING) protein, which is located on the endoplasmic reticulum (ER) membrane.
Activated STING then recruits and activates TANK-binding kinase 1 (TBK1) and IκB kinase (IKK), leading to the phosphorylation of interferon regulatory factor 3 (IRF3) and nuclear factor κB (NF-κB).
Phosphorylated IRF3 and NF-κB translocate to the nucleus and induce the expression of type I interferons and pro-inflammatory cytokines, including IFN-α and IFN-β.
Inflammatory Response:
Activation of the cGAS-STING pathway leads to the production of various pro-inflammatory cytokines and chemokines, such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interleukin-1 beta (IL-1β).
These inflammatory mediators contribute to the recruitment and activation of immune cells, amplifying the immune response against pathogens or cellular stress.
Autophagy Regulation:
STING activation can trigger autophagy, a cellular process involved in the degradation of intracellular pathogens, damaged organelles, and protein aggregates.
Autophagy serves as a mechanism to eliminate cytosolic DNA and suppress excessive inflammatory responses triggered by cGAS-STING activation.
Cell Death:
Activation of the cGAS-STING pathway can induce various forms of programmed cell death, including apoptosis, pyroptosis, and necroptosis.
These cell death pathways help to eliminate infected or damaged cells, contributing to host defense and tissue homeostasis.
DNA Damage Response:
The cGAS-STING pathway can influence the DNA damage response by regulating DNA repair mechanisms and genomic stability.
Activation of STING has been linked to the induction of DNA damage repair pathways, such as homologous recombination and non-homologous end joining.
Senescence:
Activation of the cGAS-STING pathway has been implicated in cellular senescence, a state of irreversible growth arrest associated with aging and various pathological conditions.
Senescent cells exhibit increased expression of cGAS and STING, suggesting a potential role for the pathway in driving senescence-associated inflammation and tissue dysfunction.
Immune Cell Activation:
The cGAS-STING pathway modulates the activation and function of immune cells, including dendritic cells, macrophages, and T cells.
Activation of STING in dendritic cells enhances antigen presentation and T cell priming, promoting adaptive immune responses against pathogens or tumor cells, which actually can take a turn for the worst (more in a bit on that).
Tumor Immunity:
The cGAS-STING pathway plays a critical role in the detection of tumor-derived DNA and the activation of anti-tumor immune responses. If this pathway gets dysregulated, you are in trouble.
Metabolic Regulation:
Emerging evidence suggests that the cGAS-STING pathway may influence cellular metabolism, including mitochondrial function and lipid metabolism.
STING activation has been shown to regulate mitochondrial dynamics, reactive oxygen species (ROS) production, and lipid accumulation, impacting cellular energetics and metabolic homeostasis.
Neurological Diseases:
Dysregulation of the cGAS-STING pathway has been implicated in neuroinflammatory diseases and neurodegenerative disorders, such as stroke, multiple sclerosis, and Alzheimer's disease.
Excessive activation of STING in neurons or glial cells can trigger neuroinflammation, neuronal damage, and cognitive impairment.
Autoimmune Diseases:
Aberrant activation of the cGAS-STING pathway has been linked to autoimmune diseases, including systemic lupus erythematosus (SLE), Aicardi-Goutières syndrome (AGS), and STING-associated vasculopathy with onset in infancy (SAVI).
Enhanced sensing of self-DNA or aberrant nucleic acid metabolism can lead to chronic activation of the cGAS-STING pathway, driving autoinflammation and tissue damage in autoimmune conditions.
X. Some Chronic Inflammation and Autoimmune Disorders Activated by cGAS STING:
Systemic Lupus Erythematosus (SLE):
In SLE, activation of the cGAS-STING pathway by self-DNA leads to the production of type I interferons and pro-inflammatory cytokines, causing the disease to progress and become worse.
Aicardi-Goutières Syndrome (AGS):
AGS is a rare autoimmune disorder characterized by severe neurological abnormalities and elevated levels of interferon-alpha.
Mutations in genes encoding nucleases involved in DNA metabolism, such as TREX1 and SAMHD1, lead to the accumulation of self-DNA, which activates the cGAS-STING pathway, triggering an aberrant immune response.
STING-Associated Vasculopathy with Onset in Infancy (SAVI):
SAVI is a rare autoinflammatory disease caused by gain-of-function mutations in the TMEM173 gene, encoding STING. Constitutive activation of STING results in excessive production of type I interferons and pro-inflammatory cytokines, leading to vasculopathy, skin lesions, and systemic inflammation resembling systemic lupus erythematosus.
STING-Associated Interferonopathy (SAINT):
SAINT is a recently described autoimmune disorder caused by mutations in TMEM173, resulting in constitutive activation of the STING pathway.
Chilblain Lupus (Chilblain Lupus Erythematosus CLE):
Chilblain lupus is a subtype of cutaneous lupus erythematosus characterized by painful erythematous lesions on acral areas of the body, triggered by cold exposure.
Activation of the cGAS-STING pathway has been implicated in the pathogenesis of chilblain lupus, with increased expression of interferon-regulated genes observed in affected skin lesions.
Other Autoimmune Disorders:
The cGAS-STING pathway is involved in the pathogenesis of other autoimmune diseases, including rheumatoid arthritis, psoriasis, and inflammatory bowel diseases, AIDP, autoimmune neurodegenerative, and more.
XII. Autoimmune demyelinating polyneuropathy (AIDP) is a neurological disorder characterized by damage to the myelin sheath of peripheral nerves due to an autoimmune response. Dysregulation of this pathway has been implicated in other autoimmune and neuroinflammatory diseases as well.
If the spike protein, LPS (from E Coli Endotoxin from which the plasmids were “grown in”—shout out to Dr. Pain), or DNA plasmid contamination activated this pathway, this is the potential mechanisms for it:
Immune Activation:
Activation of the cGAS-STING pathway by cytosolic DNA can lead to the production of type I interferons and pro-inflammatory cytokines, initiating and amplifying immune responses.
In AIDP, autoimmune mechanisms target peripheral nerves, leading to inflammation and demyelination.
Dysregulated activation of the cGAS-STING pathway may contribute to the perpetuation of immune-mediated damage in ADP.
Neuroinflammation:
Chronic activation of the cGAS-STING pathway in the peripheral nervous system (PNS) can lead to neuroinflammation, characterized by the infiltration of immune cells, release of inflammatory mediators, and damage to nerve tissues.
This inflammatory cascade may exacerbate demyelination and neuronal injury in AIDP, causing disease progression (see positive feedback loops above).
Interferon Signaling:
Elevated levels of type I interferons, which are produced downstream of cGAS-STING activation, have been observed in various autoimmune and neuroinflammatory conditions, including multiple sclerosis (MS) and Guillain-Barré syndrome (GBS), which are demyelinating disorders affecting the central and peripheral nervous systems, respectively.
Similarly, dysregulated interferon signaling may play a role in the pathogenesis of AIDP.
Genetic Factors:
While most cases of ADP are considered idiopathic, genetic predisposition may contribute to the development of autoimmune reactions against peripheral nerves.
Mutations in genes associated with the cGAS-STING pathway or related immune pathways could influence an individual's susceptibility to AIDP or modulate disease severity.
XI. Other factors for the continuation of cGAS STING pathway activation and autoimmune continue to attack the body—not just feedback loops:
Persistent Activation:
Even if exogenous DNA, spike protein, or LPS from endotoxins are not continuously introduced into the body, a single exposure or a series of exposures to foreign DNA, spike, or LPS can trigger a prolonged or chronic activation of the cGAS-STING pathway.
Residual DNA:
Exogenous DNA fragments, such as pieces of linearized plasmid DNA from lipid nanoparticles in the modRNA “vaccines” may persist within tissues or immune cells even after the initial exposure has ceased. There is no guarantee these will be degraded by the body—they can also bind to proteins and last awhile (more on that later).
These residual DNA fragments can continue to activate the cGAS-STING pathway and perpetuate immune responses, contributing to the persistence of inflammation and autoimmune reactions.
Cross-Reactivity:
In some cases, the immune system may develop memory responses to specific DNA sequences, leading to cross-reactivity with self-DNA or related sequences.
This molecular mimicry phenomenon can perpetuate autoimmune responses triggered by exogenous DNA, even in the absence of continuous exposure.
Secondary Stimuli:
In addition to direct activation by exogenous DNA, the cGAS-STING pathway can also be stimulated by secondary signals released during tissue damage, inflammation, or infection.
These secondary stimuli can amplify immune responses initiated by exogenous DNA and sustain the activation of the pathway over time.
Environmental Persistence (within the human body):
Exogenous DNA may persist in the environment or within microbial communities, serving as a potential source of ongoing exposure and immune activation. For example, microbial DNA present in the gut microbiota or environmental contaminants can continually stimulate the cGAS-STING pathway, contributing to chronic inflammation and immune dysregulation.
(ask your favorite “gastro” doctor if they know how to remove it—can they remove the potential DNA from YOU? The answer is …NOOOOOOOOOOOOOOOOO. Once the cGAS STING is activated, there is no going back—even in the gut—NO matter what any person says. This is one of many hills I will die on. Once the feedback loop is activated, it is now active. Unless, you want to be the FIRST PERSON in history on the planet to reverse it?)
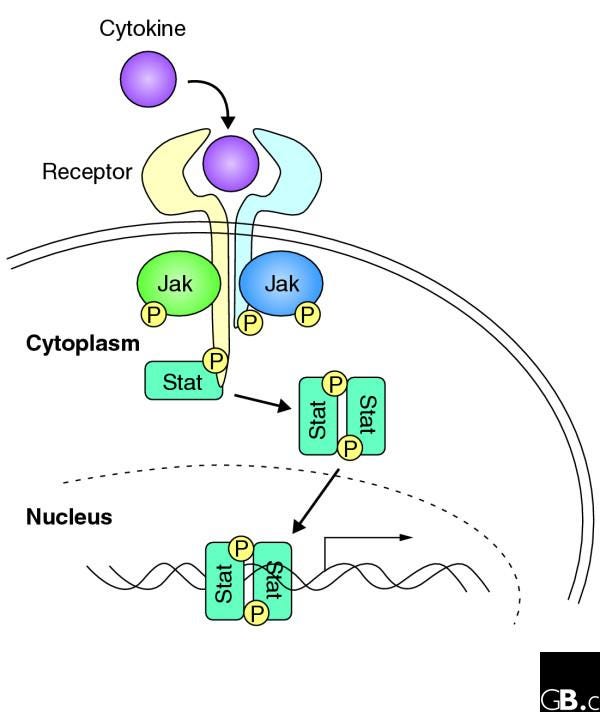
XIII. The cGAS STING and Type 1 Interferons (more in-depth):
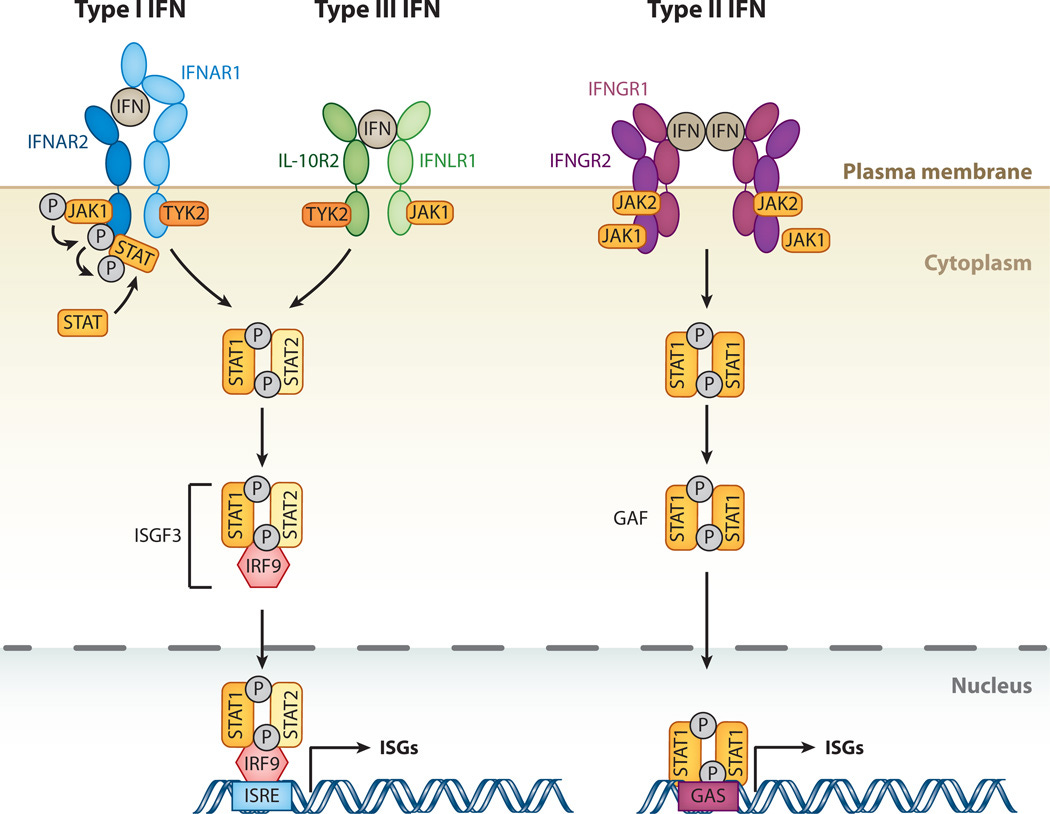
Type I Interferon Signaling Cascade:
Type I interferons bind to their cognate receptors, IFNAR1 and IFNAR2, on the surface of target cells, initiating a signaling cascade that culminates in the activation of transcription factors, such as STAT1 and STAT2 (Signal Transducer and Activator of Transcription 1 and 2).
Phosphorylated STAT1 and STAT2 form heterodimers and translocate to the nucleus, where they associate with IRF9 (Interferon Regulatory Factor 9) to form the ISGF3 (Interferon-Stimulated Gene Factor 3) transcriptional complex.
ISGF3 binds to specific DNA sequences known as interferon-stimulated response elements (ISREs) in the promoters of ISGs, inducing their transcription.
Interferon-Stimulated Genes:
ISGs encompass a diverse array of genes involved in various aspects of innate and adaptive immunity, antiviral defense, apoptosis, cell cycle regulation, and inflammatory responses.
Some ISGs encode proteins that directly participate in amplifying the cGAS-STING signaling pathway or modulating its activity, thereby creating a positive feedback loop that sustains immune responses.
Examples of ISGs Involved in Amplifying the cGAS-STING Pathway:
a. Interferon-Inducible Protein with Tetratricopeptide Repeats (IFITs):
- IFIT family members, including IFIT1, IFIT2, IFIT3, and IFIT5, are induced by type I interferons and have been implicated in enhancing innate immune responses to viral infections.
- IFIT proteins can interact with viral nucleic acids, inhibit viral replication, and modulate host antiviral defenses.
- Some IFIT proteins have been shown to directly interact with components of the cGAS-STING pathway, such as cGAS or STING, to enhance their activity and promote the production of type I interferons and other inflammatory mediators.
b. Interferon-Induced Protein with Tetratricopeptide Repeats (IFITMs):
- IFITM family members, including IFITM1, IFITM2, and IFITM3, are interferon-inducible transmembrane proteins with broad-spectrum antiviral activity.
- IFITM proteins inhibit viral entry into host cells by disrupting viral fusion and trafficking, thereby restricting viral replication and spread.
- IFITM3, in particular, has been implicated in regulating the cGAS-STING pathway and promoting the production of type I interferons in response to viral infection.
XIV. Some ISGs that directly amplify the cGAS-STING signaling pathway creating a positive feedback loop that sustains immune responses:
IFI16 (Interferon Gamma Inducible Protein 16):
IFI16 is a DNA sensor protein belonging to the PYHIN (PYD and HIN domain-containing) family. It can directly bind to cytosolic DNA and activate the cGAS-STING pathway, leading to the production of type I interferons and inflammatory cytokines.
IFI16 can also interact with STING and enhance its signaling activity, amplifying the cGAS-STING pathway.
IFIH1 (Interferon-Induced Helicase C Domain-Containing Protein 1, also known as MDA5):
IFIH1 is a cytosolic RNA sensor that detects viral RNA and activates the type I interferon response. While IFIH1 primarily recognizes RNA, it has also been implicated in the recognition of DNA viruses and stimulation of the cGAS-STING pathway in certain contexts.
Activation of IFIH1 can lead to the upregulation of type I interferons and other inflammatory mediators, contributing to immune responses.
IFI35 (Interferon-Induced Protein 35):
IFI35 is an interferon-induced protein that has been implicated in the regulation of innate immune responses. While its exact function in the context of cGAS-STING signaling is not fully understood,
IFI35 expression is induced by type I interferons and may contribute to the amplification of the immune response by modulating signaling pathways downstream of cGAS-STING activation.
IFI44 (Interferon-Induced Protein 44):
IFI44 is another interferon-inducible protein that has been implicated in antiviral defense and immune regulation.
It is upregulated in response to type I interferons and viral infections and may play a role in modulating immune responses by affecting signaling pathways involved in cGAS-STING activation and downstream effector functions.
ISG15 (Interferon-Stimulated Gene 15):
ISG15 encodes a ubiquitin-like protein that can be covalently conjugated to target proteins in a process known as ISGylation.
ISG15 conjugation has been implicated in the regulation of innate immune signaling pathways, including the cGAS-STING pathway. ISG15ylation of proteins involved in cGAS-STING signaling may modulate their activity and contribute to the amplification of immune responses.
XV. Potential ways to disrupt the cGAS STING pathway:
STING Inhibitors:
Small molecule inhibitors targeting the STING protein directly have been developed and studied as potential therapeutics for autoimmune and inflammatory diseases.
These inhibitors interfere with STING activation and downstream signaling, thereby suppressing the production of type I interferons and inflammatory cytokines.
Examples include STING antagonists such as H-151 and C-176.
cGAS Inhibitors:
Inhibition of the cGAS enzyme, which initiates the cGAS-STING signaling pathway by sensing cytosolic DNA, represents another potential therapeutic strategy. Small molecule inhibitors targeting cGAS activity or DNA binding have been investigated in preclinical studies and have shown promise in attenuating immune responses and inflammation. However, developing selective and potent cGAS inhibitors remains a challenge. They are not being used at this time. Research is still ongoing.
Interferon Signaling Blockade:
Inhibiting the downstream signaling pathways activated by type I interferons could also disrupt the positive feedback loop and dampen immune responses. Small molecule inhibitors targeting Janus kinases (JAKs), which are involved in interferon receptor signaling, have been developed and approved for the treatment of autoimmune diseases such as rheumatoid arthritis.
These drugs can suppress the production of type I interferons and downstream inflammatory mediators.
ISG15ylation Inhibitors:
Targeting ISG15ylation, a post-translational modification induced by type I interferons, represents another potential strategy for modulating cGAS-STING signaling and immune responses.
Small molecule inhibitors of the ISG15 conjugation pathway have been explored in preclinical studies and may offer therapeutic benefits by attenuating immune activation and inflammation.
Anti-inflammatory Agents:
Drugs with broad anti-inflammatory properties, such as corticosteroids or nonsteroidal anti-inflammatory drugs (NSAIDs), may also help mitigate inflammation associated with cGAS-STING activation.
While these agents do not specifically target the cGAS-STING pathway, they can suppress the production of inflammatory mediators downstream of pathway activation. Of course these come with several risks.
More in depth info on these potential treatments:
JAK Inhibitors:
Janus kinase (JAK) inhibitors, such as tofacitinib, baricitinib, and upadacitinib, are approved for the treatment of autoimmune diseases such as rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis.
These drugs work by inhibiting JAK enzymes involved in interferon receptor signaling, thereby reducing the production of pro-inflammatory cytokines downstream of type I interferon activation.
While they do not target the cGAS-STING pathway directly, they can suppress immune responses and inflammation associated with cGAS-STING activation.
Anti-TNF Agents:
Tumor necrosis factor-alpha (TNF-α) inhibitors, including drugs like adalimumab, infliximab, and etanercept, are widely used for the treatment of inflammatory diseases such as rheumatoid arthritis,
Crohn's disease, and psoriasis. By blocking TNF-α, these medications suppress inflammation and immune responses downstream of cGAS-STING activation, albeit indirectly.
Interferon Antagonists:
While not yet approved for clinical use in autoimmune diseases, research is ongoing into the development of interferon antagonists that can specifically target type I interferon signaling pathways.
These drugs aim to block the effects of excessive interferon signaling observed in conditions associated with cGAS-STING activation, such as systemic lupus erythematosus (SLE) and Aicardi-Goutières Syndrome (AGS).
Immunomodulatory Agents:
Drugs with immunomodulatory properties, such as hydroxychloroquine and methotrexate, are commonly used in the treatment of autoimmune diseases.
While their precise mechanisms of action are not fully understood, they can dampen immune responses and inflammation through various mechanisms, potentially including modulation of cGAS-STING signaling or downstream pathways.
XVI. Myocarditis (I am going out of order. Oh well. It’s like Pulp Fiction).
Myocarditis:
Myocarditis is an inflammatory condition characterized by inflammation of the myocardium, the muscular tissue of the heart.
Autoimmune mechanisms and dysregulated immune responses play a role in the pathogenesis of the disease.
Activation of the cGAS-STING pathway by DNA or self-DNA released during tissue damage may contribute to immune activation and inflammation in myocarditis.
Studies have shown that activation of the cGAS-STING pathway can exacerbate myocardial injury and worsen outcomes in myocarditis.
Order of Operations (spike or LPS would do it too):
1. Exogenous DNA Plasmid Transfection:
Exogenous DNA plasmids, are introduced into cardiomyocytes via a lipid nanoparticle. it is important to note, I have posted threads on this on twitter—the LNP does not get to every cell in the body, that is a bold faced lie. Studies have shown that the cardiomyocyte is DIFFICULT to transfect, and it requires a 10:1 molar ratio of positively charged ionizable lipids to negatively charged nucleic acids. Not every vial of “vaccine” has that, but the ones with more DNA plasmid contamination, absolutely do, and in this way, it shifts the zeta potential more negative too. Stop the lies out there and the nonsense. Ionizable Lipid Nanoparticle-Mediated Delivery of Plasmid DNA in Cardiomyocytes
Data overrides.
Plasmid Uptake:
Cardiomyocytes take up the exogenous DNA plasmids, which contain foreign DNA sequences that are recognized as non-self by the cellular machinery.
2. cGAS-STING Pathway Activation:
Recognition of Exogenous DNA:
Within the cytosol of transfected cardiomyocytes, the cGAS protein detects the presence of exogenous DNA, specifically the foreign DNA sequences from the plasmids.
Activation of cGAS:
Binding of exogenous DNA to cGAS triggers a conformational change in the protein, activating its enzymatic activity. cGAS catalyzes the synthesis of cyclic GMP-AMP (cGAMP) from ATP and GTP.
3. STING Activation and Signaling:
STING Recruitment: The synthesized cGAMP binds to STING, inducing a conformational change in STING and promoting its oligomerization.
STING Translocation:
Activated STING translocates from the endoplasmic reticulum (ER) to perinuclear puncta, where it serves as a signaling platform.
4. Downstream Signaling Cascade:
TBK1 Activation: Oligomerized STING recruits TANK-binding kinase 1 (TBK1) to perinuclear puncta, leading to TBK1 activation.
Phosphorylation of IRF3:
Activated TBK1 phosphorylates interferon regulatory factor 3 (IRF3), facilitating its dimerization and nuclear translocation.
Type I Interferon Production: Nuclear IRF3 induces the expression of type I interferons (IFN-α and IFN-β) and other pro-inflammatory cytokines.
5. Immune Response and Myocarditis Development:
Pro-inflammatory Cytokine Production: Type I interferons and other cytokines produced as a result of cGAS-STING pathway activation initiate an inflammatory response within cardiomyocytes and the surrounding myocardium.
Immune Cell Infiltration:
The release of pro-inflammatory cytokines attracts immune cells, such as macrophages and T cells, to the site of inflammation in the heart.
Tissue Damage and Myocarditis: Prolonged activation of the cGAS-STING pathway and the resulting immune response contribute to myocardial tissue damage and the development of myocarditis, characterized by inflammation and dysfunction of the heart muscle.
Aortic Dissection:
Aortic dissection is a life-threatening condition characterized by the separation of the layers within the aortic wall, leading to the formation of a false lumen and potential rupture of the aorta.
Inflammation and immune responses can contribute to disease progression and complications.
Activation of the cGAS-STING pathway by DNA damage or cellular stress in the aortic wall can trigger inflammatory responses and promote tissue remodeling and degeneration.
The cGAS-STING pathway promotes inflammation and matrix degradation in aortic dissection.
https://threadreaderapp.com/thread/1731755293527146940.html
The presence of cytosolic DNA in aortic tissues from patients with sporadic ascending thoracic AAD, showed immune system activation, as a response to dsDNA that should not be there. In patients w/ AAD, cytosolic dsDNA and STING pathway activation were detected in specific cell types, like smooth muscle cells in aortic tissues.
Smooth muscle cells impact the structural integrity of the aortic wall.
Order of operations:
Presence of exogenous dsDNA in aortic tissues, from sources such as damaged nuclei, mitochondria or DNA plasmid) is recognized by cellular "sensors" called the STING pathway. The STING pathway then activates the immune system as a response to the presence of genetic material that should not be floating around in the cytoplasm. This can occur all over the body, but right now we are focusing on rapid onset lethal aortic dissection. Immune system activation leads to the presence of interferons and macrophages, and because the STING pathway is activated, a rapid inflammatory response and condition occurs, and in this case, it is happening in the heart, in the aorta. Macrophages are "activated" and rush to the area.
The smooth muscle cells inside the wall of the aorta in the heart, then release THEIR OWN dsDNA as a response to being inflamed. This is a direct result of inflammation and damage being done by the immune attack on the body's own aorta and heart.
Macrophages then "engulf" the dsDNA (also known as double stranded DNA—plasmid DNA—also known as ODN) that is getting kicked out of the smooth muscle cells, which is different dsDNA than the dsDNA which kicked off this whole domino effect.
Then subsequent activation of STING pathway in macrophages contributes to downstream effects, including MMP-9 production and extracellular matrix (ECM) degradation.
Note: MMP-9 is an enzyme that plays a role in the degradation of extracellular matrix (ECM), which is a part of tissues that provides structural support.
The production of MMP-9 by macrophages contributes to the degradation of the ECM, thus remodeling and dissection of the aorta. This sequence drives AAD.
This includes smooth muscle cell (SMC) injury, inflammation, and ECM degradation--causing a lethal situation.
Just to be clear, the initial activation of STING before smooth muscle cell involvement with that area releasing it's OWN dsDNA are two separate parts of the domino of events, the cascade that kicks off this entire mechanisms. It can and does occur in people without an external dna contamination event.
However, exogenous dsDNA entering cells in the heart, should plausibly activate this same pathway.
In order for the heart, and the cardiomyocyte to be "transfected", a lipid nanoparticle would have to have a molar ratio of 10:1, that is 10:1 ratio of ionizable lipids to dsDNA plasmid contamination combined with modRNA to enter the area of that heart, especially the cardiomyocyte.
"Ionizable Lipid Nanoparticle-Mediated Delivery of Plasmid DNA in Cardiomyocytes"
A MAJOR concern would be, if this occurred after vaccination, and if an LNP was loaded with dsDNA and made its way to the heart, the cardiomyocyte, and namely, the aorta.
An autopsy case report of aortic dissection after mRNA COVID-19 vaccination: correspondence
XVII. How to detect cGAS STING pathway activation in tissue: various methods.
I have met with many doctors over video from around the global on different continents, in different time zones, people with current and debilitating “vaccine” injuries, parents of those with “vaccine” injuries, and those who lost children, relatives, and friends.
I know there is a huge consensus that the DNA plasmid integration caused all of these injuries, and I have seen many on Twitter, whether scientist, military person, doctor, or former of those things or combos say that “justice is coming” and that they are going to prove mutations in DNA are the cause of all of the injuries.
I have also met with multiple staff from the main law firms in these cases, including the head lawyers.
First, many went on the “non GMO” kick.
Then, many went on the SV40 kick. DNA will integrate without that. The negative charge is a NLS on it’s own, and so is the DNA itself. This is proven.
Now, it’s the “mutation” kick.
There is NO doubt that mutation has occurred, and genetic integration—that is already being proven.
But what about stroke?
Clots?
infection from LPS? Necrosis from LPS?
AAD?
https://threadreaderapp.com/thread/1720442166843994608.html
MG?
AIDP?
Myocarditis?
Who is doing cGAS STING pathway studies?
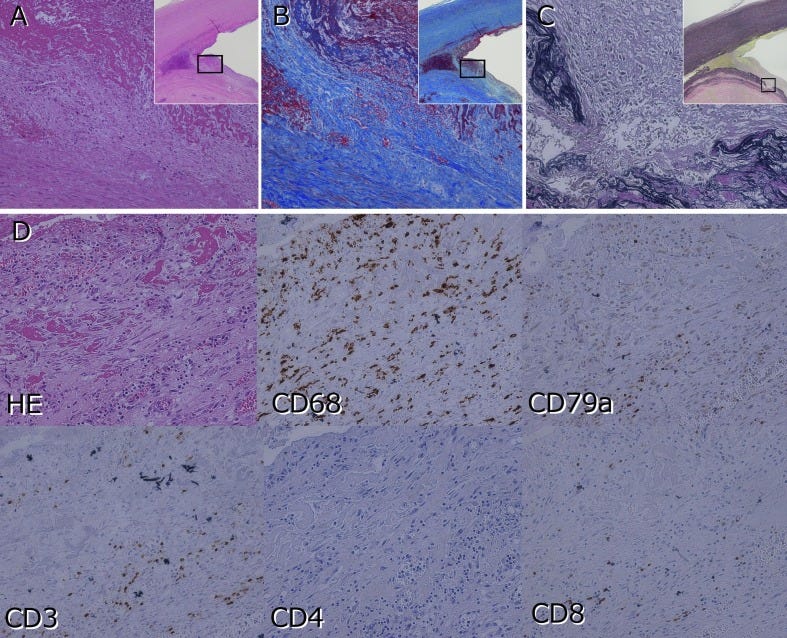
Overview of AIDP and other demyelination events: nerve biopsy tissue testing:
Histological Examination:
Nerve biopsy samples can be subjected to histological examination to evaluate the presence of characteristic pathological features associated with AIDP, such as demyelination, inflammation, axonal damage, and fibrosis.
Special stains, immunohistochemistry, and electron microscopy may be employed to visualize and characterize structural abnormalities in nerve tissue. Immunohistochemical staining of nerve biopsy samples can be performed to detect immune cell infiltrates, inflammatory cytokines, and markers of immune activation within the nerve tissue. Specific antibodies targeting proteins associated with the cGAS-STING pathway, such as cGAS, STING, type I interferons, and inflammatory cytokines, can be used to assess pathway activation and immune responses in affected nerves.
Nerve biopsy samples can undergo molecular analyses, including gene expression profiling, quantitative polymerase chain reaction (qPCR), and next-generation sequencing (NGS), to quantify mRNA levels of genes involved in the cGAS-STING pathway and immune responses.
Changes in gene expression patterns associated with pathway activation, inflammation, and neurodegeneration can be evaluated to understand molecular mechanisms underlying AIDP.
Transmission electron microscopy can provide ultrastructural insights into nerve tissue morphology, myelin integrity, axonal pathology, and the presence of intracellular structures associated with immune activation, such as mitochondrial alterations or cytoplasmic DNA aggregates.
TEM can complement histological and immunohistochemical analyses by revealing subcellular changes associated with ADP and cGAS-STING pathway activation.
DNA-Protein Interactions:
Use techniques such as chromatin immunoprecipitation (ChIP) or DNA-affinity capture assays to identify proteins that interact with ODNs in cellular extracts or tissue samples.
By immunoprecipitating protein-DNA complexes using antibodies specific to target proteins (e.g., cGAS, STING), you can isolate ODN-bound proteins and analyze their identities and functions.
DNA-DNA Interactions:
Employ methods such as electrophoretic mobility shift assays (EMSA) or DNA pull-down assays to investigate interactions between ODNs and other DNA molecules, including endogenous genomic DNA or exogenous DNA fragments.
Assess the formation of DNA complexes and their potential effects on the stability, conformation, or accessibility of DNA regions, including those involved in cGAS-STING pathway activation.
Cellular Signaling Assays:
Develop cellular assays to evaluate the functional consequences of ODN interactions on the cGAS-STING pathway and immune responses.
This may involve transfecting cells with ODNs of interest and measuring downstream signaling events, such as activation of transcription factors (e.g., IRF3, NF-κB), production of type I interferons and pro-inflammatory cytokines, or induction of antiviral responses.
Immunofluorescence and Confocal Microscopy:
Utilize immunofluorescence staining and confocal microscopy to visualize subcellular localization of ODNs and their potential colocalization with proteins associated with the cGAS-STING pathway (e.g., cGAS, STING).
Assess intracellular distribution patterns and potential sites of interaction between ODNs and pathway components.
Next-Generation Sequencing.
qPCR panels targeting genes involved in the cGAS-STING pathway, immune responses, inflammation, and neurodegeneration.
chromatin immunoprecipitation (ChIP), electrophoretic mobility shift assays (EMSA), and DNA-affinity capture assays may enable the detection of protein-DNA complexes, including those involving exogenous ODNs
Some proteins, such as transcription factors (TFs) and DNA repair enzymes, may form stable complexes with DNA molecules, including exogenous ODNs. The stability of these complexes depends on factors like the strength of protein-DNA interactions and cellular context.
Examples of stable protein-DNA interactions include TFs like NF-κB and AP-1, which can form lasting complexes with DNA sequences.
Exogenous ODNs may persist within cells or tissues if they are retained or integrated into genomic DNA.
However, rapid degradation or elimination from cells may limit the detectability of protein-ODN interactions over time.
DNA binding proteins that might still be present in nerve biopsies:
cGAS (Cyclic GMP-AMP Synthase):
cytosolic DNA, including exogenous ODNs, and catalyzes the production of cyclic GMP-AMP (cGAMP), which activates the STING pathway.
STING
Acts as a central adaptor protein in the cGAS-STING pathway, facilitating downstream signaling upon binding to cGAMP generated by cGAS.
Various TFs may interact with DNA fragments, including exogenous ODNs, to regulate gene expression. Examples include NF-κB, AP-1, IRF3, and STAT1, which are involved in immune and inflammatory responses.
Proteins involved in DNA repair pathways, such as those responsible for base excision repair (BER), nucleotide excision repair (NER), and mismatch repair (MMR), may interact with exogenous ODNs to address DNA damage or aberrant structures.
Histones and chromatin-modifying enzymes regulate chromatin structure and accessibility, potentially influencing the binding of proteins to DNA fragments, including ODNs.
IRFs are a family of TFs that regulate the expression of interferon-stimulated genes (ISGs) and play key roles in antiviral and immune responses activated by cytosolic DNA.
Proteins involved in inflammatory signaling pathways, such as cytokines (e.g., TNF-α, IL-6) and chemokines, may be induced in response to DNA fragments, contributing to local inflammation and immune activation.
Various DNA-binding proteins, such as high-mobility group (HMG) proteins, architectural transcription factors, and DNA-binding domains of other proteins, may interact with exogenous ODNs and modulate their biological effects.
Besides cGAS, other nucleic acid sensors, such as Toll-like receptors (e.g., TLR9), may recognize exogenous DNA fragments and initiate innate immune responses, potentially leading to the formation of protein-DNA complexes.
Proteins involved in apoptotic pathways, such as caspases and apoptosis-inducing factors (AIFs), may interact with DNA fragments, including those derived from exogenous ODNs, in the context of DNA damage or cell death.
Proteins involved in cellular stress responses, such as heat shock proteins (HSPs) and DNA damage response proteins (e.g., ATM, ATR), may participate in the recognition and processing of exogenous DNA fragments.
Proteins involved in epigenetic modifications, such as DNA methyltransferases (DNMTs) and histone acetyltransferases (HATs), may interact with DNA fragments, influencing chromatin structure and gene expression in response to exogenous ODNs.
You could also measure what the researchers in the cGAS STING study are measuring:
quantitative difference of amount of circulating mtDNA: d quantitative differences between the 3 groups concerning the circulating mtDNA, the circulating total DNA, by the qPCR technique: by specific primer sequences to identify the origin of the DNA (mitochondrial or nuclear). Results will be expressed by the -2∆∆Ct method ("fold increase") compared to the control.
Cytokine response: to find quantitative differences between the 3 groups concerning the cytokine response by Luminex®: in pg/ml or in ng/ml depending on the cytokines
inflammatory / dysimmune response of cytokines:
cGAS-STING pathways autophagy, NOD2, intestinal mucins, integrins, cadherins by RNAseq type transcriptomic analysis in Transcript Per Million (TPM).
Difference of DNA methylation by Methyl-Seq:
Activation (phosphorylation) of the components of the cGAS-STING pathway (proteins cGAS, p-CGAS, STING,p-STING,TBK1, p-TBK1,IRF-3, p-IRF-3) by Western-Blot
plasma DNase activity in Kunitz unitz
quantitative differences in microbial distribution by pyrosequencing RNA 16s of the microbiota at the fecal level (what are the people testing poop looking at, exactly?)
Colonic STING-specific mRNA at the colonic level
origin of the DNA (mitochondrial or nuclear). Results will be expressed by the -2∆∆Ct method ("fold increase") compared to the control.
cytoplasmic presence of STING by histology, immunohistochemical staining and optical microscopy analysis. Results will be qualitative (present/absent; localization) and quantitative based on optical density (pourcentage of positive cells)
cytoplasmic presence of cGAS by histology, immunohistochemical staining and optical microscopy analysis. Results will be qualitative (present/absent; localization) and quantitative based on optical density (pourcentage of positive cells)
cytoplasmic presence of nuclear DNA by histology, immunohistochemical staining and optical microscopy analysis. Results will be qualitative (present/absent; localization) and quantitative based on optical density (pourcentage of positive cells)
Cytoplasmic presence of mitochondrial DNA by histology, immunohistochemical staining and optical microscopy analysis. Results will be qualitative (present/absent; localization) and quantitative based on optical density (pourcentage of positive cells)
This might be one of my last stacks for a time due to medical concerns. We'll see what happens. Maybe I'll be posting more if surgery is occurring for sure. Please send photos of pets.
References:
https://www.researchgate.net/figure/The-dichotomous-roles-of-STING-dependent-signaling-Exogenous-DNA-or-self-DNA-is-sensed_fig1_331716555
https://www.researchgate.net/figure/cGAS-STING-pathway-Exogenous-DNA-from-dying-cell-tumor-cell-virus-and-bacteria-and_fig4_342373258
Advances in cGAS-STING Signaling Pathway and Diseases
Updated roles of cGAS-STING signaling in autoimmune diseases
The triggers of the cGAS-STING pathway and the connection with inflammatory and autoimmune diseases
What role of the cGAS-STING pathway plays in chronic pain?
https://www.researchgate.net/figure/Pathogenesis-of-immune-mediated-neuropathies-Onset-of-AIDP-can-be-preceded-by-an_fig1_333922230
Regulation of cGAS and STING signaling during inflammation and infection
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9550576/
Cytosolic DNA-STING-NLRP3 axis is involved in murine acute lung injury induced by lipopolysaccharide
The gut microbiota prime systemic antiviral immunity via the cGAS-STING-IFN-I axis
https://pubmed.ncbi.nlm.nih.gov/7993128/#:~:text=There%20are%20three%20types%20of,non%2Dsensitized%20lymphocytes%20with%20mitogens.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4313732/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC545791/
https://thaidj.org/index.php/JHS/article/view/12795