I started researching Nattokinase many moons ago, and initially published this on June 8th, to look at the pathways after hearing multiple medical professionals including it in their treatment protocol.
Nattokinase: An Oral Antithrombotic Agent for the Prevention of Cardiovascular Disease
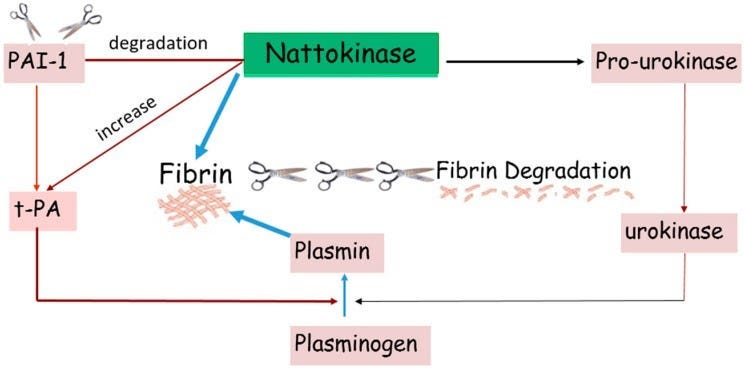
“Mechanism of Action. Nattokinase dissolves blood clots by directly hydrolyzing fibrin and plasmin substrate. It converts endogenous prourokinase to urokinase (uPA). It also degrades plasminogen activator inhibitor (PAI-1) and increases the level of tissue plasminogen activator (t-PA).”
Nattokinase will now be referred to as NK.
”NK can break down blood clots by directly hydrolyzing fibrin and plasmin substrate, converts endogenous prourokinase to urokinase (uPA), degrades PAI-1 (plasminogen activator inhibitor-1), and increases tissue plasminogen activator (t-PA) which supports fibrinolytic activity (Figure 3: Mechanism of action) [2]. Unlike common fibrinolytic proteases, such as t-PA and uPA, which can produce various side effects such as bleeding, NK exhibits little to no side effects. Studies also indicate that an oral administration of NK can be absorbed by the intestinal tract [3,4]. NK exhibits strong fibrinolytic activity after intraduodenal absorption. These characteristics make NK a versatile and potent fibrinolytic enzyme that can be used to combat blood clots. “
Bringing it down to street level:
NK is a substance that can break down blood clots in a few different ways. It can directly break down the proteins that hold the clot together, convert other substances in the body into enzymes that break down clots, and decrease the levels of a protein that stops clot breakdown. It also increases the activity of another enzyme that helps break down clots. Unlike other similar substances that can cause bleeding and other side effects, NK has very few side effects. Studies have shown that when NK is taken by mouth, it can be absorbed by the intestines and still work effectively. These characteristics make NK a powerful and versatile substance that can be used to treat blood clots.
NK works by hydrolyzing (chemically breaking down) the fibrin and plasmin substrate directly. This means that NK can break the bonds between the proteins in the clot, leading to its breakdown. By breaking down the fibrin, NK promotes the dissolution of the clot and restores blood flow in the affected area.
Does it break down proteins itself? No. Not according to the current studies.
”Nattokinase has a strong ability to breakdown thrombi and fibrin. Even a single dose of NK has been reported to result in fibrinolysis via the cleavage of cross-linked fibrin [10]. In that study, 12 healthy, young males were randomly administered a single capsule of NK (2000 FU). The antithrombin concentration in their blood increased significantly two hours after the oral consumption of the NK capsule. FDP fragments and d-dimers were observed four and six hours after NK administration, respectively, and factor VIII activity declined four hours after NK ingestion. The results of this study indicated that multiple different pathways may be involved in NK fibrinolysis and anti-coagulation activity.”
Thrombi:
Thrombi, also known as blood clots, are clumps of blood that form and stick together inside blood vessels. They usually form as a protective response to prevent excessive bleeding when there is an injury or damage to a blood vessel.
Thrombi can be problematic when they form inappropriately or when they fail to dissolve naturally. These abnormal blood clots can block blood flow in the affected blood vessel, leading to various complications depending on their location. For example, a thrombus in a deep vein of the leg can cause deep vein thrombosis (DVT), while a thrombus in a coronary artery can result in a heart attack.
Nattokinase, as mentioned earlier, is a substance that has the ability to break down thrombi by targeting the proteins, particularly fibrin, that hold the clots together. By breaking down the fibrin and reducing the size of the thrombus, nattokinase helps to restore blood flow and prevent further complications associated with blood clot formation.
Translation:
Nattokinase is a substance that is very effective at breaking down blood clots and fibrin, which is a protein involved in clot formation. Even a single dose of nattokinase has been shown to break down fibrin by cutting it apart. In a study involving 12 healthy young men, they were given a single capsule of nattokinase to take by mouth. Two hours after taking the capsule, the concentration of a protein called antithrombin in their blood significantly increased. Four hours later, fragments called FDP and six hours later, d-dimers were observed, which are markers indicating that the blood clot was breaking down. Also, the activity of a protein called factor VIII decreased four hours after taking nattokinase. These results suggest that nattokinase works in different ways to break down blood clots and has anticoagulant effects by affecting multiple pathways in the body.
Fibrinolysis: lysis means the breaking of something.
Fibrin:
Fibrin is a protein that plays a crucial role in the formation of blood clots. When there is an injury or damage to a blood vessel, the body initiates a process called coagulation or clotting to prevent excessive bleeding. Fibrin is formed as a result of this process.
Think of fibrin as a sticky mesh or net that forms at the site of an injury. It helps to trap blood cells and platelets, forming a clot that stops bleeding. This mesh of fibrin provides a scaffolding for healing and allows the body's repair mechanisms to take place. Over time, as the injury heals, the fibrin mesh is broken down and dissolved, allowing the blood vessels to return to normal function.
However, in some cases, blood clots can form when they are not needed or dissolve improperly, leading to potential health issues. Certain conditions and factors, such as genetic predisposition, prolonged immobility, or certain diseases, can increase the risk of excessive clot formation. In such cases, medications or natural substances like nattokinase can be used to help break down the fibrin and prevent or dissolve these unwanted blood clots.
It is being stated that nattokinase can break up the spike protein.
Degradative Effect of Nattokinase on Spike Protein of SARS-CoV-2
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9458005/
Abstract:
”The coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged as a pandemic and has inflicted enormous damage on the lives of the people and economy of many countries worldwide. However, therapeutic agents against SARS-CoV-2 remain unclear. SARS-CoV-2 has a spike protein (S protein), and cleavage of the S protein is essential for viral entry. Nattokinase is produced by Bacillus subtilis var. natto and is beneficial to human health. In this study, we examined the effect of nattokinase on the S protein of SARS-CoV-2. When cell lysates transfected with S protein were incubated with nattokinase, the S protein was degraded in a dose- and time-dependent manner. Immunofluorescence analysis showed that S protein on the cell surface was degraded when nattokinase was added to the culture medium. Thus, our findings suggest that nattokinase exhibits potential for the inhibition of SARS-CoV-2 infection via S protein degradation.”
“In this study, we showed that the protease activity of nattokinase contributes to the degradation of S protein. Nattokinase has a degrading effect on not only S proteins but also ACE2 in host cells. The protease specificity of nattokinase would be low, because GAPDH, a housekeeping protein, was also degraded simultaneously in the in-vitro evaluation of nattokinase mixed with cell lysate (Supplemental Figure; Figure S2). On the other hand, when added to cells, it does not show any effect on cell viability and is expected to act as a protective agent on the cell surface. Further analysis of the degradation products of nattokinase using mass spectrometry is needed for understanding the proteolysis effects.”
To determine if the breakdown effect of nattokinase on the spike protein is due to its enzyme activity, the researchers performed experiments using heat treatment and protease inhibitors. When nattokinase was heated at a high temperature (100 °C) for a short period, its ability to break down the spike protein was lost. This suggests that the enzymatic activity of nattokinase is necessary for its degradative effect.
Additionally, the researchers added a mixture of protease inhibitors to the nattokinase. The results showed that the presence of these inhibitors prevented nattokinase from breaking down the spike protein. Specifically, a combination of inhibitors known as protein cocktail III, which includes AEBSF HCl, aprotinin, and leupeptin, was particularly effective in blocking nattokinase activity.
Nattokinase contains specific conserved amino acids (building blocks of proteins) called Ser-His-Asp (Asp32, His64, and Ser221), which are characteristic of the subtilisin family of serine proteases. The crystal structure of nattokinase closely resembles that of a specific subtilisin enzyme from the bacteria B. subtilis DB104. This suggests that nattokinase, like subtilisin enzymes, functions as a serine protease.
The researchers further investigated the effects of nattokinase by using cell lysates expressing the receptor-binding domain (RBD) of the spike protein and ACE2, the cellular receptor for the virus. When nattokinase was incubated with these cell lysates, the bands corresponding to the RBD and ACE2 proteins disappeared, indicating that nattokinase successfully cleaved and degraded these proteins.
In summary, the study suggests that nattokinase has a degradative effect on the spike protein is indeed due to its enzymatic activity as a serine protease. The specific amino acids in nattokinase enable it to cleave and break down the spike protein, potentially interfering with the ability of the SARS-CoV-2 virus to infect cells.
Let’s look at this paragraph and evaluate it and compare to other proteins.
”Nattokinase contains specific conserved amino acids (building blocks of proteins) called Ser-His-Asp (Asp32, His64, and Ser221), which are characteristic of the subtilisin family of serine proteases. The crystal structure of nattokinase closely resembles that of a specific subtilisin enzyme from the bacteria B. subtilis DB104. This suggests that nattokinase, like subtilisin enzymes, functions as a serine protease.”
Serine protease:
An enzyme that plays a role in breaking down proteins in our bodies. Enzymes are like tiny molecular machines that carry out specific tasks, and serine proteases specialize in cutting proteins at specific locations. Think of proteins as long chains made up of smaller building blocks called amino acids. Sometimes, certain proteins need to be modified or broken down for various reasons. Serine proteases have a unique structure that allows them to recognize specific sites on proteins and cleave them at those points, effectively cutting the protein chain into smaller fragments.
It's similar to using a pair of scissors to cut a long piece of string into smaller pieces. In this analogy, the string represents the protein, and the scissors represent the serine protease. The serine protease recognizes a specific spot on the protein, like a target, and cuts it at that location, resulting in the protein being broken down into smaller fragments. In the context of nattokinase's activity on the spike protein of SARS-CoV-2, the serine protease activity of nattokinase allows it to cut and break down the spike protein, potentially interfering with the virus's ability to infect cells and cause illness.
”and serine proteases specialize in cutting proteins at specific locations.”
Now we are going to talk about things that are NOT in that study.
Serine proteases have a specific region in their structure called the active site. This active site is designed to bind to and interact with specific sequences of amino acids in proteins. These specific sequences are known as recognition sites or cleavage sites.
The recognition sites can vary depending on the specific serine protease and the protein it is targeting. Each serine protease has a preference for certain amino acid sequences. For example, one serine protease might recognize a sequence of amino acids like "Lys-Arg," while another might recognize "Asp-Glu." These recognition sites typically consist of a few amino acids in a specific order.
When a serine protease encounters a protein with the matching recognition site, it binds to that site, creating a temporary interaction. Once bound, the serine protease uses its unique chemical properties to break the peptide bond that holds two adjacent amino acids together within the protein chain. This cleavage results in the separation of the protein into two smaller fragments.
To visualize this process, imagine a necklace made of beads, where each bead represents an amino acid in a protein chain. The serine protease acts like a pair of scissors that can only cut at specific locations, represented by the recognition sites. When the scissors encounter a specific sequence of beads that matches its cutting preference, it cuts the necklace at that point, creating two smaller pieces.
In the case of nattokinase and the spike protein of SARS-CoV-2, the serine protease activity of nattokinase allows it to recognize and bind to specific sites on the spike protein. These binding interactions enable nattokinase to cleave the spike protein at those sites, leading to the breakdown of the protein into smaller fragments. This cleavage of the spike protein may disrupt its function and potentially hinder the virus's ability to infect cells.
It's important to note that the specific recognition sites and cleavage preferences of serine proteases can vary, and different serine proteases may have different target proteins and cleavage sites.
Here is an extensive list of proteins that serine proteases can potentially cleave:
Fibrinogen (involved in blood clotting)
Prothrombin (involved in blood clotting)
Factor V (involved in blood clotting)
Factor VII (involved in blood clotting)
Factor VIII (involved in blood clotting)
Factor IX (involved in blood clotting)
Factor X (involved in blood clotting)
Factor XI (involved in blood clotting)
Factor XII (involved in blood clotting)
Thrombin (cleaves fibrinogen to form fibrin)
Plasminogen (involved in fibrinolysis)
Plasmin (active form of plasminogen, involved in fibrinolysis)
Tissue plasminogen activator (t-PA, involved in fibrinolysis)
Urokinase-type plasminogen activator (uPA, involved in fibrinolysis)
Matrix metalloproteinases (MMPs) - enzymes involved in extracellular matrix remodeling
Collagen (structural protein in the extracellular matrix)
Elastin (structural protein in the extracellular matrix)
Gelatin (partially degraded collagen)
Proinsulin (precursor of insulin)
Prohormone convertases (involved in hormone processing)
Cytokines (immune signaling proteins)
Interleukins (a type of cytokine)
Growth factors (involved in cell growth and development)
Neuropeptides (small signaling molecules in the nervous system)
Enzymes involved in digestion:
Trypsin
Chymotrypsin
Elastase
Carboxypeptidases
Enteropeptidase
Pancreatic lipase
It is not known what else nattokinase can cleave, because the studies have not been performed.