Preprint: Patterson/Yale Purification Methods MAY Have ALTERED PATIENT MONOCYTE PHENOTYPES BEFORE TESTING, Leading to DECREASED Classical and INCREASED Non-Classical MONOCYTES as ARTIFACTS in the DATA
CYTOKINE PROFILES MAY ALSO BE IMPACTED
Some background and a timeline of how I discovered this might be happening.
Here is the pre-print:
Re-evaluating Monocyte Subset Inflammatory Profiles, Methodologies, and Potential Implications for Clinical Immune Monitoring in Long Covid and Post Vaccine Syndrome Populations
Link: https://zenodo.org/records/15783690
Image source
I do not discount anyone suffering, or those who have died from COVID, long COVID or PVS (post “vaccine” syndrome), or those who have suffered extreme illness, injury, have lost life, or those with family members who have suffered and lost life from any of this. All of this hits very close to home for me, which is why I am so passionate about it, and why I keep going.
Besides speaking out on social media on the mechanisms of harm for COVID vaccines, I started coming up with a possible drug design (last year) to combat spike protein in the human body that will hopefully eradicate the spike protein production at the source—not just cleaning up the blood, but something that hits tissues too—complete destruction of the source of spike protein manufacturing at the cellular level.
I knew from reading the Patterson et al. and Yale study that monocytes were impacted, and from several other studies, that other cell types are involved in spike protein expression (as targets for the drug).
Image source
I met with the patent lawyer for the first time this year, February 2025.
I had already put together a “pitch deck”, and was quoting the Yale Study and Patterson et al., and other references in the drug design proposal and funding pitch for the drug production and pre-clinical work (with funders waiting to jump in at phase one who are local to me).
As I began consulting more with the patent lawyer and CDMOs, and putting together a more detailed proposal, I wanted more references on different cell types to make certain the targets were hit, including but not limited to monocytes, and to make certain the drug has little off target effects.
When I began looking for more references on cell types that are producing spike protein, especially monocytes, I found a study on lupus and monocytes.
I discovered in the study, while looking at monocytes of patients with lupus, that the investigators, according to this study that researched different methods that other researchers may use to separate the monocytes from whole blood (Ficoll purification), that the physical separation methods, handling, AND storage methods, can CHANGE the MONOCYTE PHENOTYPE BEFORE TESTING EVEN BEGINS.
This means that the final test results may be inaccurate, not just for the monocytes that CHANGE PHENOTYPE during the mechanical and handling processes of the MONOCYTES, but OTHER SURFACE MARKERS MAY BE CHANGING, LIKE CYTOKINES.
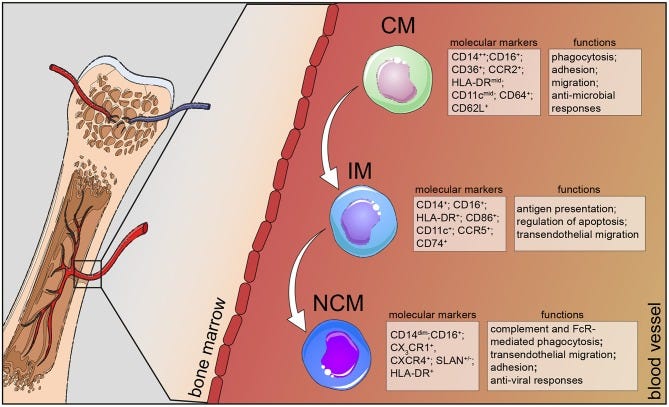
CM=Classical Monocyte
IM= Intermediate Monocyte
NCM= Non-Classical Monocyte
image source
I told two people over the phone that I had discovered this (almost a month ago now), and these two people, who are outspoken in the circles of COVID “vaccines” and injuries, told me not to put this in print, because the outcome would be, and I quote, me “getting attacked” by the original study authors and others, so I delayed going to print.
I wrote the paper (preprint) comparing the lupus study findings on testing monocyte classification and other immunological concerns, and compared the findings to the methodology and results sections of the Patterson et al. paper, and the Yale study (both have monocyte analysis and other data sets), the potential concerns and implications, and it has sat on my computer for weeks.
Then I sent some of the references to myself a couple of weeks ago (June 15).
Here are some layman’s terms explanations beneath the abstract and important graph, of what the researchers in the lupus paper found (science in the preprint), and how this might impact researchers who perform analysis on the blood (OR tissue) of patients on monocytes, and how methods of separating monocytes from the blood (use of Ficoll or Lymphoprep purification methods) might CHANGE the monocytes, because ten years ago, researchers found there were concerns of those reporting different types of monocytes found in our bodies, what they do, how they are separated from whole blood impacts their phenotype, and what they found.
Non-Classical monocytes display inflammatory features: Validation in Sepsis and Systemic Lupus Erythematous
Mukherjee, R., Kanti Barman, P., Kumar Thatoi, P. et al. Non-Classical monocytes display inflammatory features: Validation in Sepsis and Systemic Lupus Erythematous. Sci Rep 5, 13886 (2015). https://doi.org/10.1038/srep13886
Abstract
”Given the importance of monocytes in pathogenesis of infectious and other inflammatory disorders, delineating functional and phenotypic characterization of monocyte subsets has emerged as a critical requirement. Although human monocytes have been subdivided into three different populations based on surface expression of CD14 and CD16, published reports suffer from contradictions with respect to subset phenotypes and function. This has been attributed to discrepancies in reliable gating strategies for flow cytometric characterization and purification protocols contributing to significant changes in receptor expression. By using a combination of multicolour flow cytometry and a high-dimensional automated clustering algorithm to confirm robustness of gating strategy and analysis of ex-vivo activation of whole blood with LPS we demonstrate the following: a. ‘Classical’ monocytes are phagocytic with no inflammatory attributes, b. ‘Non-classical’ subtype display ‘inflammatory’ characteristics on activation and display properties for antigen presentation and c. ‘Intermediate’ subtype that constitutes a very small percentage in circulation (under physiological conditions) appear to be transitional monocytes that display both phagocytic and inflammatory function. Analysis of blood from patients with Sepsis, a pathogen driven acute inflammatory disease and Systemic Lupus Erythmatosus (SLE), a chronic inflammatory disorder validated the broad conclusions drawn in the study.”
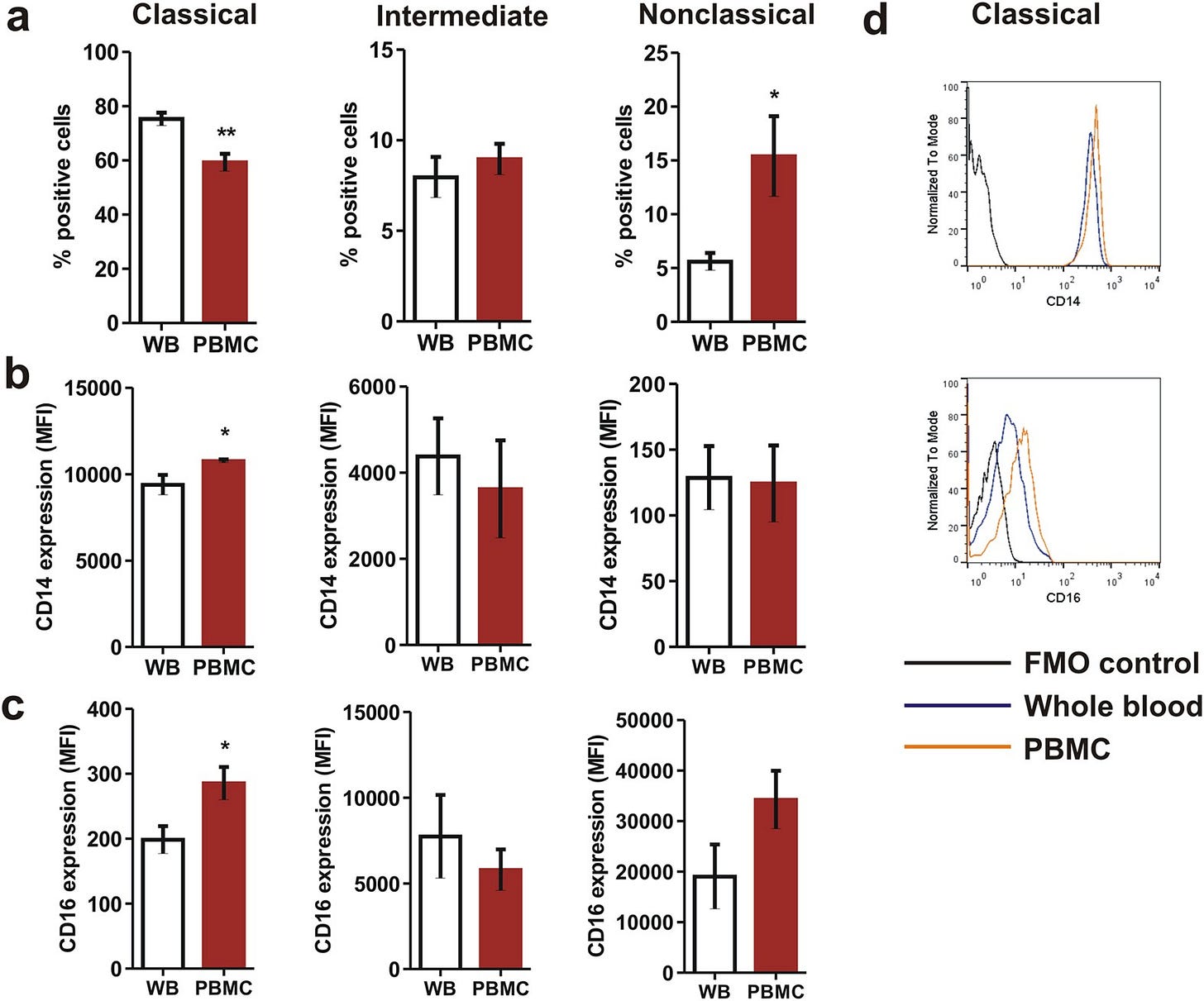
“Gradient purification of PBMCs from whole blood leads to changes in monocyte phenotype”.
Comparison of monocyte subsets between whole blood monocytes and Ficoll purified monocytes showing (a) significant decrease in proportion of ‘classical’ monocytes and increase in ‘nonclassical’ monocytes as a consequence of gradient purification that also led to increase in (b) CD14 and (c) CD16 expression on classical monocytes. Statistical significance was ascertained using a paired t-test. (d) Representative overlaid histograms showing increased expression of CD14 and CD16 on classical monocyte surface as a consequence of Ficoll purification. Data are mean ± SEM of five normal subjects. *P < 0.05, **P < 0.01. MFI: Median Fluorescence Intensity.”
As layman’s terms as it is going to get:
Researchers studying lupus and sepsis wanted to understand the different types of monocytes found in the human body. Monocytes are immune cells that help fight infections and control inflammation. Normally, monocytes are divided into three groups based on proteins on their surface: classical, intermediate, and non-classical.
Each type has different jobs in the body, like cleaning up debris, producing (or controlling) inflammation, or presenting antigens on their surface to other immune cells.
Presenting antigens means that monocytes take tiny pieces of viruses or bacteria and display them on their surface, holding up a kind of “look at me, come deal with this!” flag. You could think of it as a flag, or a signal flare. This helps other immune cells recognize what the body needs to fight things that should not be in our bodies.
However, the scientists noticed that many previous studies disagreed about which monocyte types were most inflammatory or what roles they played. When the researchers of the lupus study looked closer, they found a crucial problem: the way blood samples were prepared for testing (FICOLL method of purification) actually changed the monocytes themselves.
The way that different researchers separated monocytes out from whole blood FORCED some of the monocytes to hold up DIFFERENT FLAGS!
In this study, they compared testing monocytes directly in whole blood to testing after using purification methods like density gradients (Ficoll). They found that purification stressed the monocytes, causing them to change the proteins on their surface, meaning the monocytes changed their flags for identification.
The researchers found that gradient purification (Ficoll), and other stressors on the monocytes, while handling them and preparing them for testing, made the classical monocytes decrease in number, by quite a remarkable amount! At the same time, while the classical monocyte markers (numbers) decreased, the NON-CLASSICAL MONOCYTES INCREASED!
Some classical monocytes even started showing markers that made them look like non-classical types. This means the purification process (Ficoll) didn’t just separate the cells, the stress on the monocytes physically altered them. And by the time the researchers tested the monocytes to classify them, this purification gave inaccurate counts of classical monocytes and non-classical monocytes.
This kind of change caused by the testing method is called an artifact. It’s like a false or misleading signal (or false flag if you will) introduced by how the cells were handled in the lab, not a true reflection of what’s happening inside the body. This means that monocytes in the body, if tested right from the body, most likely would not show such a decrease in classical monocyte numbers and increases in non-classical monocyte numbers, but in the lab, it did.
Why does this matter for COVID “vaccine” injuries and long COVID?
Biologically, monocytes—including non-classical ones—do not naturally live that long. Their normal lifespan in circulation is measured in days, not months.
This needs further review.
Also, monocytes might pick up spike protein from reservoirs like long lived somatic cells and, without a better phrase, God forbid, stem cells.
Monocytes act as “clean-up” cells, phagocytosing (engulfing) these spike protein fragments as part of normal immune surveillance.
The monocyte phenotypes Patterson et al./Yale measured may, POTENTIALLY be artificially altered by purification (if they used similar purification methods to Ficoll like Lymphoprep), meaning, the populations they observed as “non-classical” might be overestimated or misidentified, while classical monocyte numbers showed a marked decrease as artifacts, and unfortunately, resulted in potentially inaccurate data sets.
If purification (Ficoll/Lymphoprep) itself makes monocytes look more “non-classical” than they really are in the bloodstream, while decreasing the number of classical monocytes in lab samples, these studies may potentially be reporting results that are partly artifacts of their lab methods rather than real reflections of what is happening in patients’ bodies.
WHAT ABOUT CYTOKINE MARKERS?
The way monocytes show their “flags” also involves cytokines—small signaling molecules that monocytes release to communicate and direct the immune response. These cytokines act like different colored flags that tell the immune system whether to ramp up inflammation or tone down.
The lupus study looked closely at the three main monocyte types: classical, intermediate, and non-classical. It showed that:
Classical monocytes mainly act like cleaners, gobbling up debris and don’t produce much inflammatory cytokines like IL-1β or TNF-α.
Non-classical monocytes are the main producers of inflammatory cytokines IL-1β and TNF-α, especially after stimulation. These cytokines promote inflammation and help alert the immune system to danger.
Intermediate monocytes produce mostly anti-inflammatory cytokines, like IL-10, which help tone down the immune response.
Importantly, the study found that common lab methods that isolate monocytes (like Ficoll purification, which is almost identical to Lymphoprep) alter these cytokine profiles. For example, purification causes the cells to change their surface markers and reduces the amount of inflammatory cytokines they produce, meaning purified samples may underestimate how inflammatory the non-classical monocytes actually are.
In more simpler terms, the lupus study showed that when monocytes go through stressful lab preparation methods like gradient purification to separate them from the blood before testing, not only do their surface markers (the flags they hold up) change, but their cytokine production may shift too. This means the inflammatory signals that were detected might be altered or even suppressed by the lab methods, giving an incomplete picture of what these cells are really doing inside the body.
This also means that if there are any tests being done outside of these two studies, then there may potentially be concerns with the data that is being given to patients.
Conclusion
When researchers report shifts in monocyte populations or cytokine production in long COVID or post-vaccine syndromes, it’s crucial to know whether the cells were tested in a way that preserves their true behavior, or if lab processing using Ficoll or Lymphoprep might have introduced misleading changes.
If the samples still exist from these studies, AND if some (at least SOME) of the original participants are willing to donate whole blood again (although it is a different time point, so now we have a variable that has changed), and the monocytes are tested in whole blood without separation, then we will know.
This is also not to say that this research is not important, because IT IS, and people suffering IS important, and finding ways to test and help people IS important.
Long term spike expression IS happening. This is not being debated here. People are suffering. This is also not contested.
However, we must know for certain what happened in these studies, and what is happening in testing panels for monocyte data, who are also receiving treatments based on these monocyte lab values.
I really hope this all gets re-evaluated using whole blood.
***NOTE***
From : https://www.stemcell.com/products/lymphoprep.html
“Reliably isolate mononuclear cells from peripheral blood, cord blood, or bone marrow with Lymphoprep™—a cost-effective alternative to Ficoll-Paque™. Use this density gradient medium for rapid, simple, and reliable cell isolation from most blood samples obtained from normal individuals and patients. You can substitute Lymphoprep™ for Ficoll-Paque™ without changing your existing protocols and achieve similar cell purity and recovery rates. This medium is fully compatible with both SepMate™ and RosetteSep™. Formulated with sodium diatrizoate (9.1% w/v) and polysaccharide (5.7% w/v), Lymphoprep™ has a density of 1.077 g/ml”
Refences
Parihar A, Eubank TD, Doseff AI. Monocytes and macrophages regulate immunity through dynamic networks of survival and cell death. J Innate Immun. 2010;2(3):204-15. doi:10.1159/000296507. Epub 2010 Mar 16. PMID:20375558; PMCID:PMC2956013.
Kapellos TS, Bonaguro L, Gemünd I, Reusch N, Saglam A, Hinkley ER, Schultze JL. Human monocyte subsets and phenotypes in major chronic inflammatory diseases. Front Immunol. 2019 Aug 30;10:2035. doi:10.3389/fimmu.2019.02035. PMID:31543877; PMCID:PMC6728754.
Mukherjee, R., Kanti Barman, P., Kumar Thatoi, P. et al. Non-Classical monocytes display inflammatory features: Validation in Sepsis and Systemic Lupus Erythematous. Sci Rep 5, 13886 (2015). https://doi.org/10.1038/srep13886
Patterson BK, Yogendra R, Francisco EB, Guevara-Coto J, Long E, Pise A, et al. Detection of S1 spike protein in CD16+ monocytes up to 245 days in SARS-CoV-2-negative post-COVID-19 vaccine syndrome (PCVS) individuals. Human Vaccines & Immunotherapeutics. 2025 May 13;[Epub ahead of print]. doi:10.1080/21645515.2025.2494934.
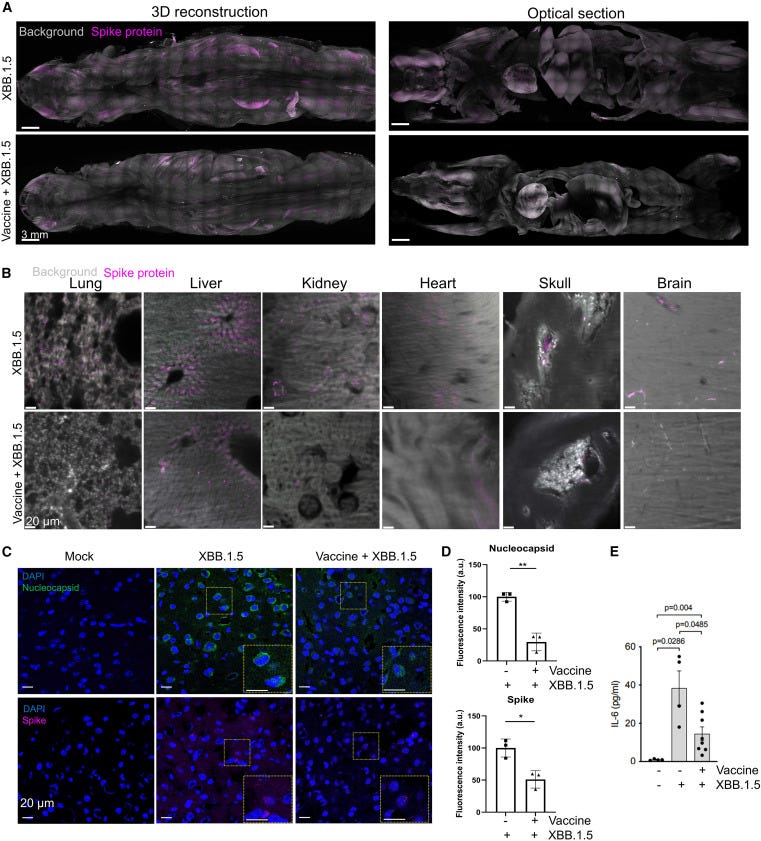
Bhattacharjee B, Lu P, Monteiro VS, Tabachnikova A, Wang K, Hooper WB, Bastos V, Greene K, Sawano M, Guirgis C, Tzeng TJ, Warner F, Baevova P, Kamath K, Reifert J, Hertz D, Dressen B, Tabacof L, Wood J, Cooke L, Doerstling M, Nolasco S, Ahmed A, Proal A, Putrino D, Guan L, Krumholz HM, Iwasaki A. Immunological and antigenic signatures associated with chronic illnesses after COVID-19 vaccination. medRxiv. 2025 Feb 18. doi:10.1101/2025.02.18.25322379.