CANCER: "COMBINED" FEEDBACK LOOP of cGAS STING AND APOBEC: DNA plasmid in LNP +DS RNA triggers cGAS STING AND induction of DNA deaminase APOBEC3A + nuclear DNA damage:
Dysregulation/ positive feedback loops of cGAS STING meets APOBEC (a DIFFERENT MULTI-HIT mechanism):
I posted this on X (twitter tonight)
I am just copying and pasting it right over (I need sleep)
Hard science time.
(this stack is important as a foundation):
A. First read substack below for cGAS STING activation--massive stack: on the RNA/LNP combo.
Here are some highlights from that:
The cGAS-STING pathway is a critical component of the innate immune system. It serves as sensor for cytosolic DNA and orchestrating the cellular response to various microbial infections, cellular stress, and DNA damage.
You could think of it like a smoke detector sensing smoke, and then activating an alarm system, that responds to danger, but worse.
cGAS is a cytosolic DNA sensor that recognizes double-stranded DNA (dsDNA) and double stranded RNA (and spike)derived from pathogens, damaged cells, or cellular debris.
(I am not going to ncontinue to type double stranded RNA for activation of cGAS or spike to save space, time, and to avoid constant redundancy)
Upon DNA binding, cGAS catalyzes the synthesis of cyclic GMP-AMP (cGAMP) from ATP and GTP.
STING (Stimulator of Interferon Genes):
STING is an endoplasmic reticulum (ER)-resident protein that serves as a signaling adaptor downstream of cGAS.
Recognition of Pathogen DNA:
cGAS detects cytosolic DNA derived from bacteria, viruses, or other pathogens.
Pathogen DNA may be released during infection, replication, or cell lysis.
DNA that is exogenous DOES include what is called ODN—the pieces of plasmid DNA that are in the current modRNA “vaccines” will also activate this pathway. Spike protein will also activate, and there are recent studies showing that DS DNA will do it too.
Upon binding to cGAMP or other cyclic dinucleotides (CDNs), STING undergoes conformational changes and translocates from the ER to perinuclear puncta, where it recruits downstream signaling effectors.
Infammatory conditions, such as autoimmune diseases, cancer, or tissue injury, can lead to the release of self-DNA or DAMPs (damage-associated molecular patterns), activating the cGAS-STING pathway in infiltrating immune cells or stromal cells.
Activation of the cGAS-STING pathway induces the expression of type I interferons and other antiviral effectors, leading to the inhibition of viral replication, clearance of infected cells, and activation of adaptive immune responses.
Dysregulation of the cGAS-STING pathway has been implicated in various autoimmune diseases, including systemic lupus erythematosus (SLE), Aicardi-Goutières syndrome (AGS), and type I interferonopathies, where aberrant activation of the pathway leads to the production of autoantibodies, tissue inflammation, and organ damage.
Inflammatory processes can lead to cell death or damage, resulting in the release of self-DNA from damaged or dying cells.
This self-DNA can further activate the cGAS-STING pathway in neighboring cells or immune cells, perpetuating the immune response.
Positive Feedback Loop:
The release of self-DNA and continued activation of the cGAS-STING pathway create a positive feedback loop, amplifying the immune response and sustaining inflammation.
This amplification mechanism can contribute to chronic inflammation and autoimmune diseases if dysregulated, including MS, AIDP, Sjögren's Syndrome, Myocarditis, and more.
The FEEDBACK LOOP: HOW the cGAS STING PATHWAY IS MAINTAINED IN THE “ON” POSITION—CONSTANT INFLAMMATION, AUTOIMMUNE ATTACK, AND WORSENING OF CONDITIONS--including CANCER EVEN IF THERE IS NO LONGER A PRESENCE OF PLASMID DNA, Double Stranded RNA, SPIKE PROTEIN, OR LPS:
Positive Feedback Loops:
Upon activation, the cGAS-STING pathway triggers the production of type I interferons (IFNs) and other inflammatory cytokines, which in turn promote the expression of interferon-stimulated genes (ISGs).
Some ISGs encode proteins that directly amplify the cGAS-STING signaling pathway or modulate its activity, creating a positive feedback loop that reinforces immune responses.
These proteins may include factors involved in signal transduction, transcriptional regulation, or post-translational modifications.
Inflammatory Signaling Cascades:
In addition to type I IFNs, other pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) can activate downstream signaling pathways, including NF-κB and MAPK pathways.
These signaling cascades further enhance the expression of inflammatory mediators, perpetuating inflammation and immune cell activation.
Chronic Tissue Damage and Inflammation:
Prolonged activation of the cGAS-STING pathway and sustained immune responses can lead to chronic tissue damage and inflammation.
Cellular stress, DNA damage, and mitochondrial dysfunction may exacerbate cGAS-STING activation, creating a cycle of tissue injury and immune activation.
Damaged tissues release danger-associated molecular patterns (DAMPs), including self-DNA, ATP, and HMGB1, which further stimulate innate immune responses and maintain inflammation.
Autoimmune Responses:
In autoimmune diseases, the cGAS-STING pathway may become dysregulated, leading to the recognition of self-DNA as foreign and the generation of autoantibodies against self-antigens. Autoantibodies, particularly those targeting nuclear antigens, can form immune complexes that activate complement cascades and recruit immune cells, perpetuating tissue inflammation and injury.
Epigenetic Regulation:
Epigenetic modifications, including DNA methylation, histone modifications, and non-coding RNA regulation, can modulate the activity of genes involved in the cGAS-STING pathway and immune responses. Epigenetic changes induced by chronic inflammation or cellular stress can contribute to the persistence of immune activation and the development of autoimmune phenotypes.
Positive Feedback Loops:
Upon activation by type I interferons (IFNs), ISGs are upregulated, many of which encode proteins involved in amplifying the cGAS-STING signaling pathway.
For example, IFN-induced proteins such as IFIT1, IFIT3, and MX1 can directly interact with components of the pathway to enhance signaling or promote downstream effector functions.
Amplification of Signaling Pathways:
Some ISGs may modulate the activity of key signaling molecules involved in the cGAS-STING pathway, such as TBK1 (TANK-binding kinase 1) and IRF3 (interferon regulatory factor 3), leading to the amplification of downstream signaling events and the sustained production of inflammatory mediators.
Inflammatory Signaling Cascades:
NF-κB Pathway:
Activation of NF-κB signaling by inflammatory cytokines such as TNF-α and IL-1β can synergize with the cGAS-STING pathway to enhance the expression of pro-inflammatory genes.
NF-κB target genes include cytokines, chemokines, and adhesion molecules that recruit immune cells to sites of inflammation and promote tissue damage.
MAPK Pathway:
Mitogen-activated protein kinase (MAPK) signaling pathways, including the ERK, JNK, and p38 MAPK pathways, can be activated by inflammatory stimuli and contribute to the regulation of gene expression, cell proliferation, and immune responses.
MAPK activation downstream of cGAS-STING signaling may further amplify inflammatory signaling cascades and immune cell activation.
Chronic Tissue Damage and Inflammation:
Cellular Stress and DNA Damage:
Persistent activation of the cGAS-STING pathway and sustained immune responses can lead to cellular stress, DNA damage, and mitochondrial dysfunction.
Accumulation of damaged cellular components and reactive oxygen species (ROS) contributes to tissue injury and inflammation, perpetuating immune activation and exacerbating tissue damage.
Loss of Self-Tolerance:
Dysregulated activation of the cGAS-STING pathway and prolonged exposure to self-DNA can lead to the breakdown of immune tolerance and the generation of autoreactive immune responses.
Autoantibodies targeting self-antigens, such as nuclear antigens in systemic lupus erythematosus (SLE), form immune complexes that drive tissue inflammation and contribute to autoimmune pathology.
Epigenetic Regulation:
DNA Methylation and Histone Modifications:
Epigenetic modifications can regulate the expression of genes involved in the cGAS-STING pathway and immune responses.
Changes in DNA methylation patterns and histone modifications may alter the accessibility of gene promoters, enhancers, and regulatory elements, influencing the transcriptional activity of key immune genes.
Cell Death:
Activation of the cGAS-STING pathway can induce various forms of programmed cell death, including apoptosis, pyroptosis, and necroptosis.
These cell death pathways help to eliminate infected or damaged cells, contributing to host defense and tissue homeostasis.
DNA Damage Response:
The cGAS-STING pathway can influence the DNA damage response by regulating DNA repair mechanisms and genomic stability.
Activation of STING has been linked to the induction of DNA damage repair pathways, such as homologous recombination and non-homologous end joining.
Senescence:
Activation of the cGAS-STING pathway has been implicated in cellular senescence, a state of irreversible growth arrest associated with aging and various pathological conditions.
Senescent cells exhibit increased expression of cGAS and STING, suggesting a potential role for the pathway in driving senescence-associated inflammation and tissue dysfunction.
Immune Cell Activation:
The cGAS-STING pathway modulates the activation and function of immune cells, including dendritic cells, macrophages, and T cells.
Activation of STING in dendritic cells enhances antigen presentation and T cell priming, promoting adaptive immune responses against pathogens or tumor cells, which actually can take a turn for the worst (more in a bit on that).
Cancer:
The cGAS-STING pathway plays a critical role in the detection of tumor-derived DNA and the activation of anti-tumor immune responses. If this pathway gets dysregulated, you are in trouble.
Inflammatory cytokines and reactive oxygen species (ROS) generated during chronic inflammation can damage cellular DNA.
This damage can lead to the formation of more cytosolic DNA fragments, perpetuating the activation of the cGAS-STING pathway.
Chronic inflammation and the resulting DNA damage can interfere with normal DNA repair processes.
Error-prone repair mechanisms may become more prevalent, increasing the likelihood of mutations and genomic instability.
DNA damage creates more cytosolic DNA fragments, which further activate cGAS.
This leads to more production of cGAMP and subsequent STING activation, maintaining a cycle of inflammation and DNA damage.
Feedback Loops in cGAS-STING Pathway
Inflammation-Induced DNA Damage:
Inflammatory cytokines, such as TNF-α and IL-6, contribute to cellular stress and DNA damage.
ROS production during chronic inflammation also damages DNA.
DNA damage can result in chromosomal aberrations and the formation of micronuclei.
Micronuclei rupture, releasing DNA into the cytosol, which is detected by cGAS.
The presence of cytosolic DNA continuously activates the cGAS-STING pathway.
This leads to sustained production of inflammatory mediators, creating a persistent inflammatory state.
Chronic inflammatory signaling can downregulate effective DNA repair pathways.
The cellular environment becomes more conducive to accumulating mutations due to error-prone repair mechanisms.
B. Now let's add in APOBEC to the equation, when talking about DNA plasmids (and spike perhaps) AND double stranded RNA--both from OUR cells (mitochondria) AND if it were present in the lipid nanoparticles inside the COVID vaccines--you have TWO sources of it now) and what that would look like if cGAS STING got dysregulated, and how that ties into APOBEC mutations:
APOBEC (Apolipoprotein B mRNA Editing Enzyme, Catalytic Polypeptide-like) enzymes are a family of cytidine deaminases involved in RNA editing and innate immune responses. Mutations or overexpression of APOBEC enzymes, especially APOBEC3, can lead to genomic instability through their off-target activity on single-stranded DNA (ssDNA), which often occurs during DNA replication or repair.
Here's how APOBEC mutations and activity relate to the cGAS-STING
Off-Target Mutagenesis:
APOBEC enzymes typically target viral genomes to induce mutations that inhibit viral replication. However, when these enzymes act on cellular ssDNA, they introduce mutations by deaminating cytosine to uracil. This off-target activity can lead to a high mutation burden, causing genomic instability and contributing to cancer development.
The uracil bases introduced by APOBEC activity are recognized as lesions by the DNA repair machinery, leading to base excision repair (BER). However, if repair is faulty or overwhelmed, this can result in double-strand breaks (DSBs), further increasing genomic instability.
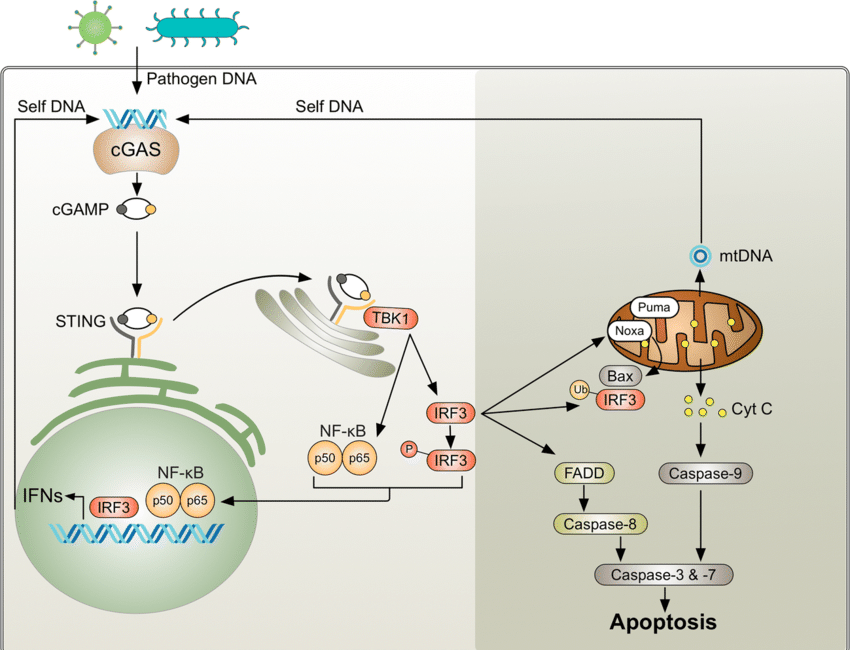
Activation by DNA Damage: The cyclic GMP-AMP synthase (cGAS) senses cytosolic DNA, which can result from cellular stress, DNA damage, or viral infection. Upon recognizing DNA, cGAS synthesizes cyclic GMP-AMP (cGAMP), a second messenger that activates the Stimulator of Interferon Genes (STING) pathway.
Activated STING triggers a signaling cascade leading to the production of type I interferons and other inflammatory cytokines, promoting an antiviral state and recruiting immune cells to the site of infection or damage.
So now, DNA damage and genomic instability caused by APOBEC enzymes can lead to the presence of cytosolic DNA fragments, which can activate the cGAS-STING pathway. This links APOBEC-induced mutagenesis and genomic instability with the activation of an innate immune response--which is activation of cGAS STING by DNA plasmids, DS RNA, spike, etc.
(FYI, this could be happening without any vaccines).
The Tumor Microenvironment: Chronic activation of the cGAS-STING pathway due to persistent DNA damage and genomic instability can contribute to an inflammatory tumor microenvironment. This can influence cancer progression and the immune response to tumors.
Summary: APOBEC-induced genomic instability can activate the cGAS-STING pathway through the generation of cytosolic DNA, linking mutagenesis and DNA damage to innate immune activation. However, cGAS STING activation can generate APOBEC genomic instability, introducing the chiekn versus the egg scenario, or, is it option THREE--it's happening in tandem, and at intersecting points.
This relationship is significant in the context of cancer development and the tumor immune microenvironment.
cGAS STING meets APOBEC.
But if we were to proceed in a more linear fashion:
DNA Damage and Cytosolic DNA:
Various factors such as oxidative stress, radiation, chemotherapy, or replication stress can cause DNA damage, resulting in the formation of double-strand breaks (DSBs).
During the repair process, fragments of DNA can end up in the cytosol. The presence of cytosolic DNA activates cGAS.
The other thigns that can activate cGAS, the smoke detector for the human body, are pices of plasmid DNA contamination, double stranded RNA (hope this was not recently found in COVID vaccines), double stranded RNA from our own body (mitochondria), or other source. Spike protein will also activate cGAS, and bacteria, so now the smoke detector is activated, and it triggers the immune response, like water raining down on a fire, but the water is going to do damage, to our cells, our house.
So once again, be it double stranded RNA, DNA plasmids, spike, or bacteria, we now have cGAS Activation:
cGAS binds to cytosolic DNA and catalyzes the synthesis of cyclic GMP-AMP (cGAMP).
STING Activation:
cGAMP binds to and activates the STING protein located on the endoplasmic reticulum membrane.
Activated STING initiates a signaling cascade involving TBK1 and IRF3, leading to the production of type I interferons (IFNs) and other inflammatory cytokines.
This is important in APOBEC in tandem with cGAS STING, like chocolate and peanut butter.
Genomic Instability and APOBEC Activity through cGAS STING ACTIVATION:
The production of type I IFNs and cytokines leads to an inflammatory response, attracting immune cells to the site of damage.
Chronic inflammation and immune responses can induce further DNA damage, OUR DNA, in OUR CELLS, and these include MITOCHONDRIA, which are going to release their own dsRNA , because "Mitochondrial double-stranded RNA triggers induction of the antiviral DNA deaminase APOBEC3A and nuclear DNA damage" --exacerbating genomic instability.
Persistent DNA damage and repair attempts increase replication stress. During this process, single-stranded DNA (ssDNA) regions become more frequent, serving as substrates for APOBEC enzymes.
ALSO, if you already have double stranded RNA within an LNP, you are ALSO going to have these acting as a substrate for APOBEC enzymes.
And now, not only is cGAS STING activated, so is APOBEC.
APOBEC Activation:
The innate immune response, particularly through interferon signaling, can upregulate APOBEC enzymes as part of the antiviral response.
APOBEC enzymes deaminate cytosine to uracil in ssDNA, introducing mutations. MORE mutations.
The cGAS-STING pathway, once activated, can contribute to genomic instability and potentially influence APOBEC activity, creating a feedback loop that drives cancer progression and impacts the tumor microenvironment. Here’s a detailed sequence of events illustrating this process:
Activation of the cGAS-STING Pathway
DNA Damage and Cytosolic DNA:Various factors such as oxidative stress, radiation, chemotherapy, or replication stress can cause DNA damage, resulting in the formation of double-strand breaks (DSBs).
During the repair process, fragments of DNA can end up in the cytosol. The presence of cytosolic DNA activates cGAS.
cGAS Activation:cGAS binds to cytosolic DNA and catalyzes the synthesis of cyclic GMP-AMP (cGAMP).
STING Activation:cGAMP binds to and activates the STING protein located on the endoplasmic reticulum membrane.
Activated STING initiates a signaling cascade involving TBK1 and IRF3, leading to the production of type I interferons (IFNs) and other inflammatory cytokines.
Genomic Instability and APOBEC Activity
Inflammatory Response:The production of type I IFNs and cytokines leads to an inflammatory response, attracting immune cells to the site of damage.
Chronic inflammation and immune responses can induce further DNA damage, exacerbating genomic instability.
DNA Repair and Replication Stress:Persistent DNA damage and repair attempts increase replication stress. During this process, single-stranded DNA (ssDNA) regions become more frequent, serving as substrates for APOBEC enzymes.
APOBEC Activation:The innate immune response, particularly through interferon signaling, can upregulate APOBEC enzymes as part of the antiviral response.
APOBEC enzymes deaminate cytosine to uracil in ssDNA, introducing mutations.
Feedback Loop and Cancer Progression
APOBEC-Induced Mutations:APOBEC-induced mutations can lead to a high mutational burden, contributing to genomic instability and creating mutations that can drive cancer progression.
Some of these mutations may inactivate tumor suppressor genes or activate oncogenes, promoting cancer development and evolution.
Further Activation of cGAS-STING:As genomic instability persists, more DNA fragments may accumulate in the cytosol, perpetuating the activation of the cGAS-STING pathway.
Impact on Tumor Microenvironment:The cGAS-STING pathway’s activation leads to chronic inflammation within the tumor microenvironment, which can support tumor growth and immune evasion.
Inflammatory cytokines can create a microenvironment that is more conducive to cancer progression by promoting angiogenesis, metastasis, and suppressing anti-tumor immune responses.
Summary
Initial DNA damage activates the cGAS-STING pathway, causing inflammation and immune responses.
Chronic activation of this pathway leads to further DNA damage and genomic instability.
Genomic instability provides substrates for APOBEC enzymes, which introduce mutations.
APOBEC-induced mutations further increase genomic instability and contribute to cancer progression.
Persistent DNA damage results in continued activation of the cGAS-STING pathway, creating a feedback loop.
The tumor microenvironment becomes inflamed and supportive of cancer progression due to chronic activation of the cGAS-STING pathway and APOBEC loop combined.
C. NOW
Let's delve deeper into the detailed sequence of events illustrating how the cGAS-STING pathway can contribute to genomic instability and influence APOBEC activity, creating a feedback loop that drives cancer progression and impacts the tumor microenvironment. We’ll start from the introduction of plasmid DNA and DOUBLE STRANDED RNA (and spike) into cells using lipid nanoparticles and proceed through the sequence of events.
cGAS STING Meets APOBEC double dose pathway for positive feedback loop and CANCER--they work TOGETHER< and INTERACT with one another.
Here's a shortened detailed sequence of events illustrating how the cGAS-STING pathway can contribute to genomic instability and influence APOBEC activity, creating a feedback loop that drives cancer progression and impacts the tumor microenvironment. We’ll start from the introduction of plasmid DNA, double stranded RNA (and spike, or possible bacteria) into cells using lipid nanoparticles and proceed through the sequence of events.
Introduction of Plasmid DNA and Double Stranded RNA
Plasmid DNA is introduced into cells via lipid nanoparticles, a common method for delivering nucleic acids into cells. This process involves encapsulating the DNA within lipid-based particles, which can fuse with cell membranes and release the DNA, AND double stranded RNA into the cytoplasm.
Recognition of Cytosolic DNA/DSRNA
The plasmid DNA released into the cytoplasm is recognized as foreign or damaged DNA by the cell’s innate immune system. The cyclic GMP-AMP synthase (cGAS) protein is a key sensor of cytosolic DNA.
cGAS binds to the cytosolic plasmid DNA, (or DS RNA) which activates its enzymatic activity. cGAS then catalyzes the synthesis of cyclic GMP-AMP (cGAMP) from ATP and GTP.
:cGAMP acts as a second messenger and binds to the Stimulator of Interferon Genes (STING) protein located on the endoplasmic reticulum (ER) membrane.
Binding of cGAMP activates STING, which then translocates from the ER to the Golgi apparatus, where it initiates downstream signaling cascades.
Signaling Cascade:
Activated STING recruits and activates TANK-binding kinase 1 (TBK1), which in turn phosphorylates and activates Interferon Regulatory Factor 3 (IRF3).
Phosphorylated IRF3 dimerizes and translocates to the nucleus, where it induces the expression of type I interferons (IFNs) and other inflammatory cytokines.
Genomic Instability and APOBEC Activity THROUGH CGAS STING:
Inflammatory Response:The production of type I IFNs and cytokines leads to an inflammatory response, attracting immune cells to the site of DNA presence.
Chronic inflammation and the immune response can cause further DNA damage, contributing to genomic instability.
Persistent DNA damage requires continuous repair. During DNA replication, especially under conditions of replication stress, single-stranded DNA (ssDNA) regions become more frequent. These ssDNA regions are susceptible to the activity of APOBEC enzymes.
🚨🚨APOBEC Activation:
The innate immune response, particularly the signaling through type I IFNs, can upregulate the expression of APOBEC enzymes. APOBEC3 enzymes, for instance, are known to be involved in antiviral responses but can also act on the host genome.
APOBEC enzymes deaminate cytosine to uracil in ssDNA, introducing mutations.
MASSIVE HYPER Feedback Loop BETWEEN CGAS STING AND APOBEC LEADING TO Cancer Progression
APOBEC-Induced Mutations ARE NOW OCCURING:
The deamination of cytosine to uracil by APOBEC enzymes results in C-to-T transitions in DNA. These mutations can accumulate, leading to a high mutational burden.
Some of these mutations can inactivate tumor suppressor genes or activate oncogenes, driving cancer progression and evolution.
NOW THESE APOBEC MUTATIONS SLAM CGAS STING RIGHT IN THE FACE LIKE A MAC TRUCK:
Further Activation of cGAS-STING:
As genomic instability persists, additional DNA fragments may be released into the cytosol, either from damaged nuclear DNA or during mitotic errors.
The continuous presence of cytosolic DNA leads to sustained activation of the cGAS-STING pathway, perpetuating the cycle of inflammation and DNA damage.
TUMOR TIME:
The chronic activation of the cGAS-STING pathway creates a pro-inflammatory tumor microenvironment. This environment can support tumor growth and facilitate immune evasion.
Inflammatory cytokines promote angiogenesis (formation of new blood vessels), metastasis (spread of cancer cells), and suppression of anti-tumor immune responses, creating a microenvironment conducive to cancer progression.
WRAPPING THIS STUDY IN (AND THEN WE WILL TIE IT ALL TOGETHER):
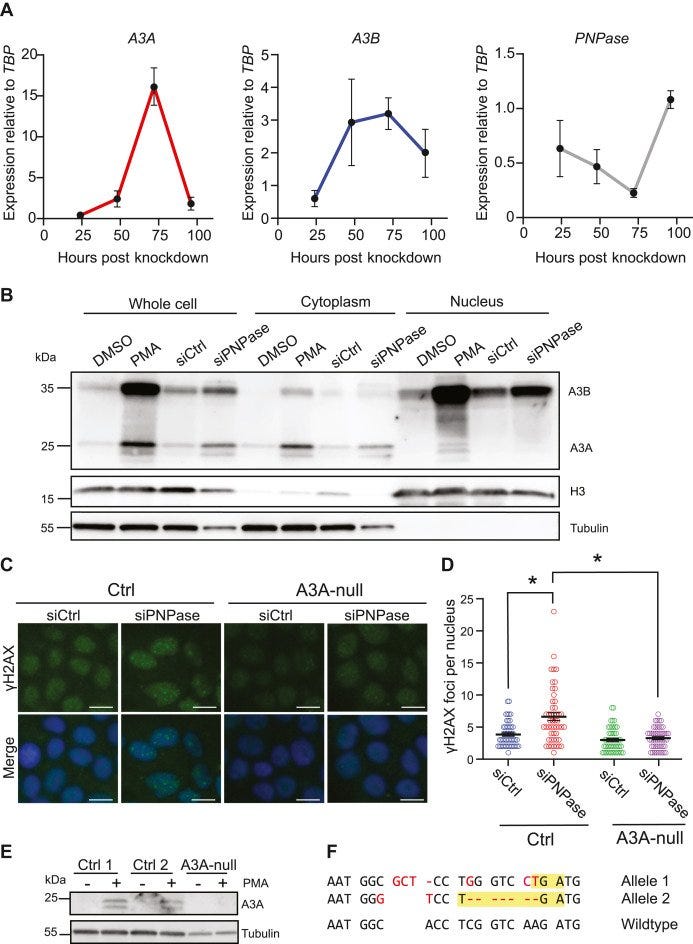
"Mitochondrial double-stranded RNA triggers induction of the antiviral DNA deaminase APOBEC3A and nuclear DNA damage"
(THE STUDY AND THEN WE COMBINE IT ALL):
Double-Stranded RNA (dsRNA) Perspective
Breakdown of the Study
Introduction to APOBEC3A:APOBEC3A is a DNA deaminase with antiviral properties.
It can be induced by viral infections and is implicated in cancer mutations.
Hypothesis on Mitochondrial dsRNA:
(NOW THIS IS IMPORTANT BECAUSE IF NOT ONLY DNA PLASMID CONTAMINATION EXISTS IN COVID MODRNA LNP "VACCINES" BUT ALSO DOUBLE STRANDED RNA, THEN WE HAVE DOUBLE TROUBLE TIME:
Leaked mitochondrial dsRNA (mtdsRNA) may trigger innate immune signaling, upregulate APOBEC3A, and cause DNA damage.
(NOT PART OF STUDY, BUT NKLP CONTAINING DOUBLE STRANDED RNA WILL DO THIS TOO, AND ACTIVATE CGAS STING SIMULTANESOULY):
Polynucleotide phosphorylase (PNPase) normally degrades mtdsRNA. Knockdown of PNPase results in mtdsRNA leakage into the cytosol.
Then the immune system is activated in this study (which can also be activated by the double stranded RNA--APOBEC is engaging with the double stranded RNA that is in the LNP, while the double stranded DNA is SIMULTANEOUSLY HITTING the cGAS STING--CREATING CHAOS in the CELLS)
mtdsRNA in the cytosol is recognized by the RIG-I-like receptor (RLR) pathway, involving RIG-I, MAVS, and STAT2.
This recognition triggers a type I interferon (IFN) response, leading to APOBEC3A upregulation.
APOBEC3A Induction and DNA Damage:
Upregulation of APOBEC3A, despite being cytoplasmic, induces nuclear DNA damage marked by elevated γ-H2AX staining.
This is now going to cause genomic instability, (with the simultaneous activation of cGAS STING causing genomic instability).
mtdsRNA-induced APOBEC3A contributes to genomic instability and mutation signatures in cancer.
APOBEC3A is upregulated transiently upon mitochondrial dysfunction, suggesting episodic bursts of mutagenesis.
Part DOS (two):
Double-Stranded RNA and Cancer
dsRNA in Cancer
APOBEC and dsRNA:
dsRNA from mitochondrial leakage or viral infections activates immune responses, upregulating APOBEC3A.
APOBEC3A’s mutagenic activity introduces C-to-T and C-to-G mutations in the genome, contributing to cancer progression.
cGAS-STING Pathway and dsRNA:
While the study focuses on RIG-I/MAVS/STAT2 in response to dsRNA, the cGAS-STING pathway typically responds to cytosolic DNA, However, double stranded RNA can ALSo activate cGAS STING, and APOBEC simultaneously, causing
dsRNA-induced inflammation and IFN responses create an environment conducive to DNA damage, indirectly activating cGAS-STING.
cGAS STING and APOBEC combined now are driving genomic instability, inflammation, immune system hgyperactivation, and a "hyper" feedback loop.
Chronic inflammation and immune responses from dsRNA presence exacerbate genomic instability, providing substrates for APOBEC enzymes.
This results in a feedback loop where genomic instability perpetuates APOBEC activity and further mutagenesis.
Part THREE (tres):
DNA Plasmid Pieces, DOUBLE Stranded RNA pieces and cGAS-STING and APOBEC :
Activation of cGAS-STING Pathway:
DNA plasmids and double strqanded RNA introduced into cells via lipid nanoparticles can be recognized as foreign DNA.
cGAS binds to this cytosolic DNA (and double stranded RNA) , synthesizing cGAMP, which then activates STING.
DOUBLE TROUBLE:
Downstream Signaling:
Activated STING triggers TBK1 and IRF3, leading to type I IFN production and an inflammatory response.
Chronic inflammation promotes DNA damage and replication stress, increasing ssDNA regions.
APOBEC Activity COMES ONLINE:
Type I IFNs upregulate APOBEC enzymes, which target ssDNA and introduce mutations.
Mutations drive cancer progression by inactivating tumor suppressors or activating oncogenes.
PART FOUR (quatro)
Double-Stranded RNA and cGAS Activation
Potential for dsRNA to Activate cGAS:hybrids.
Although cGAS primarily senses dsDNA, recent studies suggest cGAS can also recognize RNAdsRNA AND rna with DNA and might contribute to cGAS activation by causing DNA damage that releases dsDNA into the cytosol.
Consequences of cGAS Activation by dsRNA:hybrids leads to inflammation and further DNA damage.
Activation of the cGAS-STING pathway by RNAThis creates a feedback loop of genomic instability and APOBEC activity, driving cancer progression.
FULLY INTEGRATED SUMMARY: PART FIVE (CINCO):
Tying It All Together
Integrated Model:
APOBEC ACTIVITY:
Initial Trigger: dsRNA, from mitochondrial leakage or viral infections, or from double stranded RNA inside a lipid nanoparticle, activates the RIG-I/MAVS pathway, leading to type I IFN production and APOBEC3A upregulation.
APOBEC Feedback Loop:
APOBEC3A introduces mutations in ssDNA regions, contributing to genomic instability.
DNA damage from chronic inflammation releases dsDNA, activating the cGAS-STING pathway.
Now look at that again. APOBEC is getting activated by the double stranded RNA in the LNP, and activating cGAS STING, but wait!
Double stranded DNA and double stranded RNA (and SPIKE ALSO activate cGAS STING!
Mutational Landscape--cancer time (and automimmnue, to say the least!!)
dsRNA Perspective:
dsRNA-driven inflammation and immune responses create a mutagenic environment through APOBEC activity.
cGAS-STING Perspective:
DNA plasmids, double stranded RNA, and damaged nuclear DNA activate the cGAS-STING pathway, perpetuating inflammation and mutagenesis.
Interconnected Pathway--MULTI HIT FEED BACK LOOP OF cGAS STING and APOBEC:
Both dsRNA and DNA damage responses interlink, with dsRNA potentially enhancing cGAS activation indirectly through genomic instability.
FASTER CANCER PROGRESSION:
Chronic activation of immune responses by dsRNA and dsDNA together in one incredible (and frightening) feedback loop between the APOBEC feedback loop, cGAS STING feedback loop, and the two combining, creates a pro-inflammatory tumor microenvironment.
This environment supports tumor growth, angiogenesis, metastasis, and immune evasion, driven by the continuous mutational input from APOBEC enzymes.
https://sciencedirect.com/science/article/pii/S0021925823021014
sources (I combined): https://sciencedirect.com/science/article/pii/S0021925823021014… https://christiegrace.substack.com/p/cgas-sting-pathway-activation-by… https://researchgate.net/figure/The-dichotomous-roles-of-STING-dependent-signaling-Exogenous-DNA-or-self-DNA-is-sensed_fig1_331716555… https://researchgate.net/figure/cGAS-STING-pathway-Exogenous-DNA-from-dying-cell-tumor-cell-virus-and-bacteria-and_fig4_342373258… https://researchgate.net/figure/Pathogenesis-of-immune-mediated-neuropathies-Onset-of-AIDP-can-be-preceded-by-an_fig1_333922230… https://ncbi.nlm.nih.gov/pmc/articles/PMC9550576/… https://pubmed.ncbi.nlm.nih.gov/7993128/#:~:text=There%20are%20three%20types%20of,non%2Dsensitized%20lymphocytes%20with%20mitogens…. https://ncbi.nlm.nih.gov/pmc/articles/PMC4313732/… https://ncbi.nlm.nih.gov/pmc/articles/PMC545791/… https://thaidj.org/index.php/JHS/article/view/12795