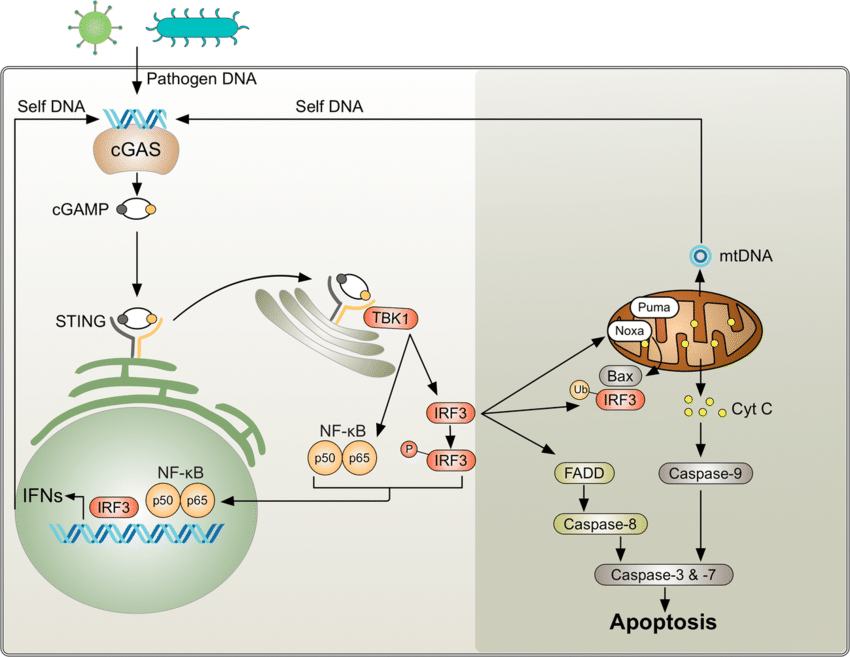
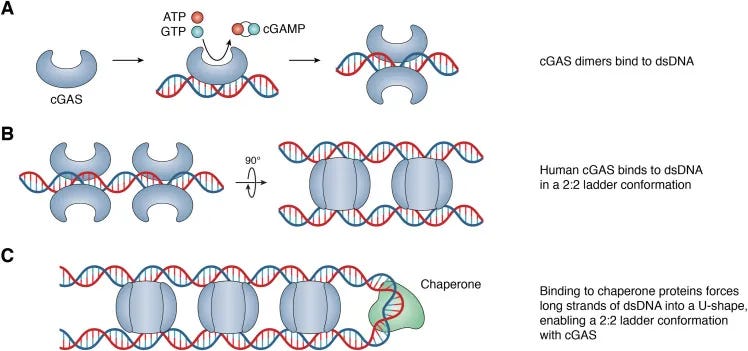
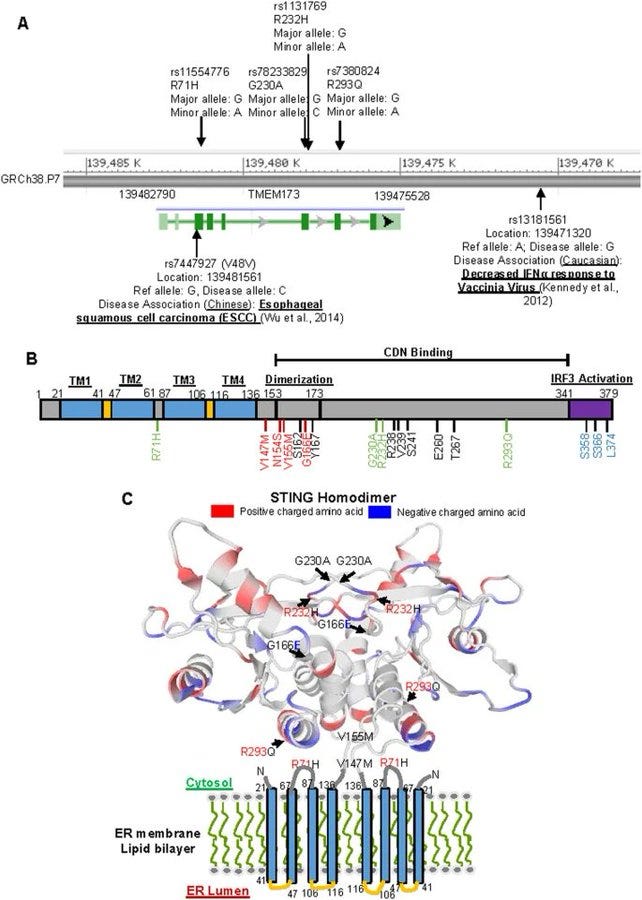
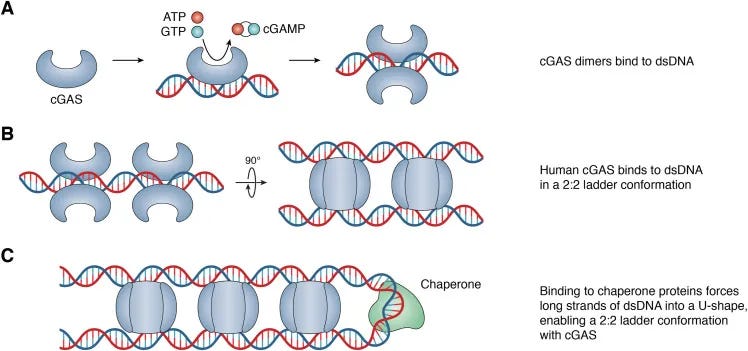
cGAS STING Interactions and Written Work
a handful more of these blocks of threads are on the way
All of these threads, and everything contained here, are a published work on Substack, and copyrighted, and protected under the Digital Millennium Copyright Act (DMCA). I do not give consent for the use of or republication of this material, under any format.
https://www.copyright.gov/dmca/
Christie Laura Grace
Subscribe
Oct 18, 2023 • 22 tweets • 7 min read • Read on X
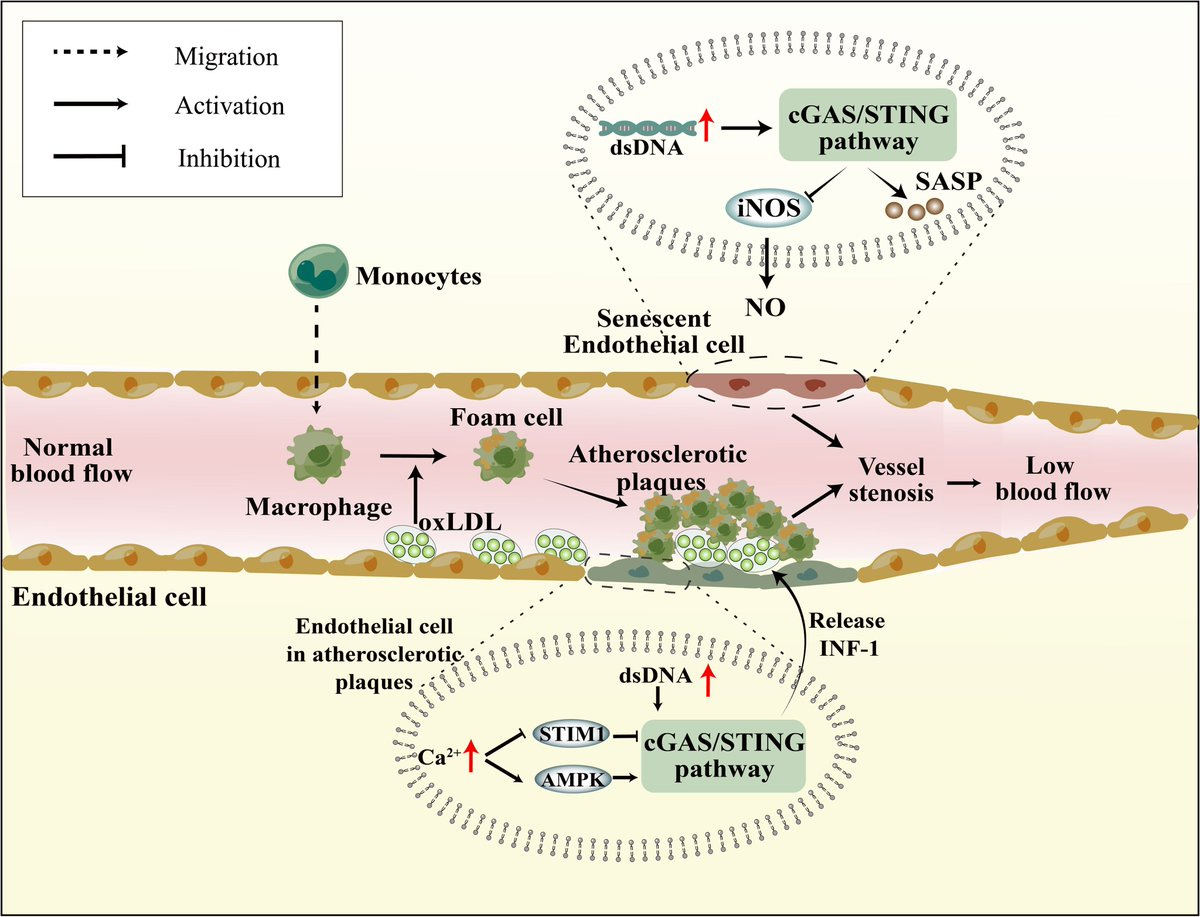
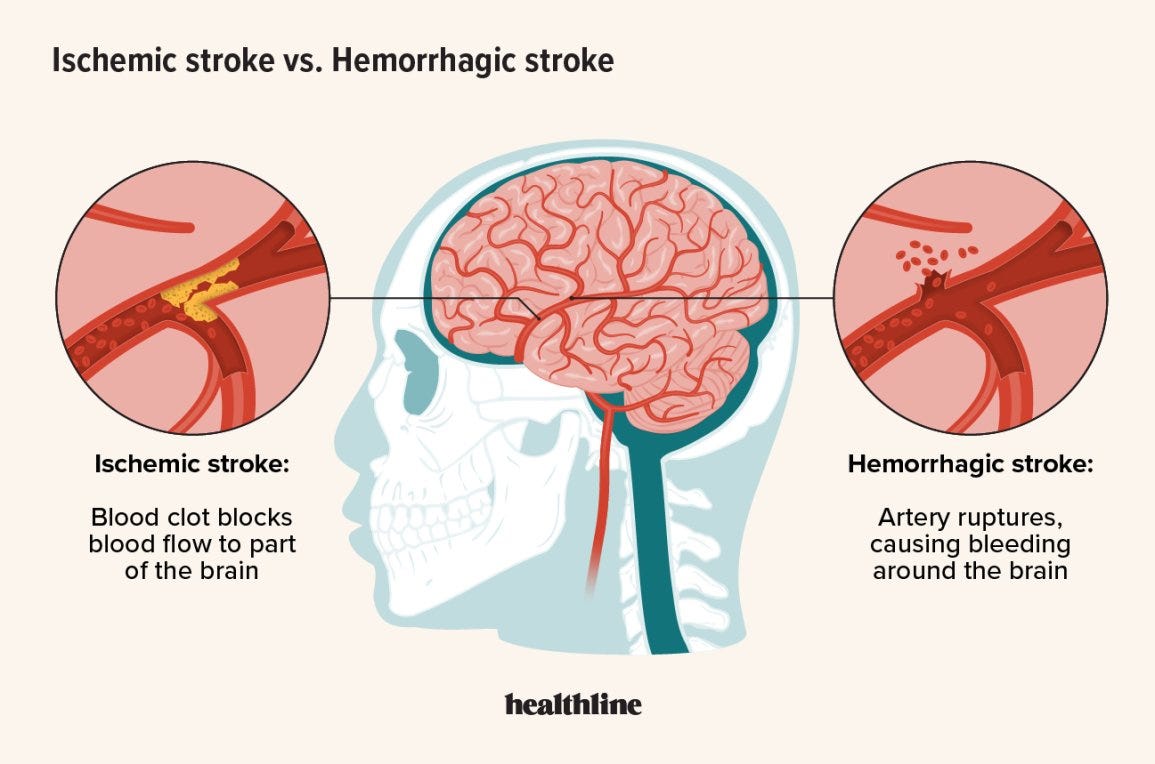
1/ 🚨 dsDNA [PLASMID CONTAMINATION IN LNP/modRNA 💉] CAN CAUSE STROKES
BREAKING STUDY PUBLISHED 10/17/2023
(possible part one--might need more threads)
THE STUDY: "Novel insight into cGAS-STING pathway in ischemic stroke: from pre- to post-disease"
doi.org/10.3389/fimmu.…
Frontiers | Novel insight into cGAS-STING pathway in ischemic stroke: from pre- to post-diseaseIschemic stroke, a primary cause of disability and the second leading cause of mortality, has emerged as an urgent public health issue. Growing evidence sugg...https://doi.org/10.3389/fimmu.2023.1275408
2/Study highlights (layman's terms coming!)
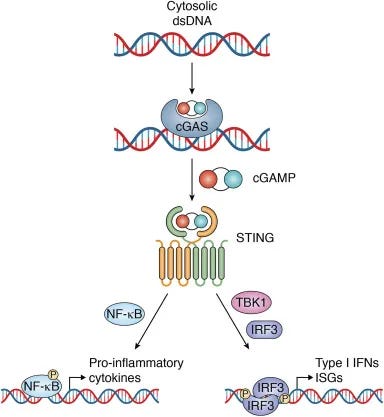
cGAS-STING pathway is mediator of inflammation in response to dsDNA.
The cGAS-STING pathway is linked with start of the neuroinflammatory response in ischemic stroke.
The cGAS-STING pathway is involved in various forms of RCD.
3/ Breakdown for "X" (brief explanation of steps):
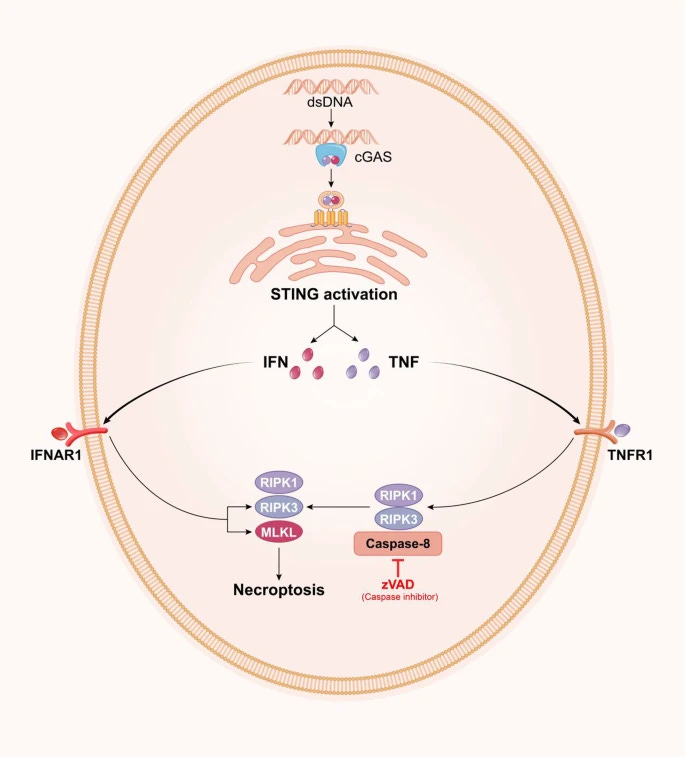
The presence of dsDNA activates the cGAS-STING pathway--part of the innate immune system. The cGAS-STING pathway detects dsDNA, which kicks off the immune and inflammation response, which leads to ischemic stroke.
4/ cGAS (Cyclic GMP-AMP Synthase) is like a sensor in your body, like a smoke detector. It detects signs of trouble. When it senses something suspicious, it sends out a signal.
STING (Stimulator of Interferon Genes) is like the alarm that goes off when the smoke detector (cGAS)
5/senses a problem. It receives the signal from cGAS and sets off a series of reactions. When STING is activated, it alerts your immune system, which is like your body's defense force. Your immune system then gears up to fight off whatever is causing the trouble (dsDNA plasmid)
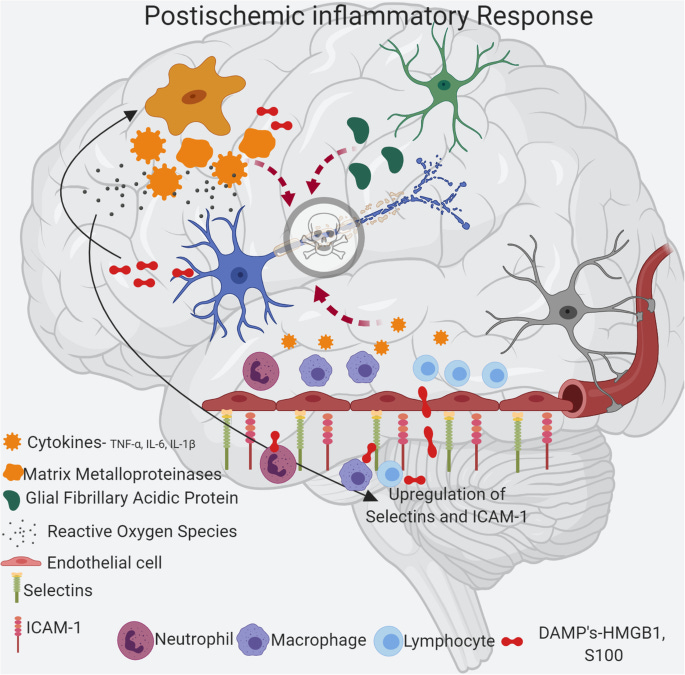
6/ Activation of the cGAS-STING pathway by dsDNA triggers neuroinflammation in the brain. Pro-inflammatory signals and activation of immune cells like microglia takes place. The brain's defense system, including immune cells like microglia (which are the brain's resident immune
7/ cells), becomes active. It's as if the brain's "security team" is mobilized to respond to the threat. The cGAS-STING releases signals that are like calls for reinforcements. These signals tell the immune cells to prepare for action and also attract other immune cells to the
8/scene. This is similar to how a siren alerts both local and additional law enforcement to a crime scene.
Microglia are like the brain's first responders. They become more active, ready to clear away any damage and deal with the threat. This activity can sometimes cause
9/ inflammation, which is the brain's way of defending itself against potential harm.
Cell Death
cGAS-STING induces different types of cell death, such as ferroptosis, pyroptosis, apoptosis, and necroptosis. These forms of regulated cell death contribute to the brain damage
10/ associated with strokes.
When the cGAS-STING is activated in response to dsDNA, it can trigger different ways for brain cells to die. It's like a "self-destruct" mechanism for cells in the brain.
Ferroptosis is a specific type of cell death where brain cells break down
11/ because of an excess of iron and certain fats in the brain. It's like the cells are rusting and falling apart.
Pyroptosis means brain cells essentially explode, releasing substances that can cause more inflammation. It's like the cells burst open, creating chaos in the brain.
12/ in apoptosis, brain cells die in a very orderly and controlled way. It's like cells committing a programmed "suicide" to prevent further damage, just like a self-destruct.
Necroptosis is a chaotic form of cell death, where cells swell up and burst, causing inflammation.
13/ It's like the cells are bursting open uncontrollably.
It's like different weapons causing different types of damage in space, making the overall situation more severe. The brain is getting hit with multiple forms of cell and tissue death, from the presence of dsDNA.
14/ Then, the immune system responds to the dsDNA by releasing inflammatory signals and attracting immune cells to the brain. This immune response can exacerbate damage in the already stressed brain tissue.
Starship Enterprise Alert:
Think of the brain as the USS Enterprise,
15/ a starship on a peaceful mission. Suddenly, the crew detects an alien intruder in the form of dsDNA.
RED ALERT! The crew activates "Red Alert" and raises shields (releasing inflammatory signals) to prepare for a possible battle.
Time to beam in reinforcements!
16/ In response to the threat, the ship calls in friendly starships (immune cells) from nearby galaxies. They hurry to assist but arrive in the middle of a cosmic battle (stroke) that's already underway.
The problem is, the space battle (stroke) is fierce, and the sudden arrival
17/of more starships (immune cells) adds to the intergalactic chaos, potentially making things worse.
So, when the immune system senses dsDNA, it's like the Enterprise detecting an alien presence and calling for backup. While this may seem like a good idea, it can actually create
18/ more interstellar turbulence in the midst of an intense space skirmish (stroke).
In non Star Trek terms, when dsDNA is detected in the brain, it triggers a response from the immune system. This response includes the release of signals that promote inflammation and
19/ the recruitment of immune cells, like microglia, to the affected area. While this reaction is intended to protect the brain, it can inadvertently worsen the situation, as it adds to the stress and damage already occurring during a stroke. In essence, the immune system's
20/ attempt to defend the brain can inadvertently contribute to the problem, potentially making the outcome of a stroke more severe.
Of course this is a real occurrence. It is not happening to everyone who receives 💉, but it is happening to some people.
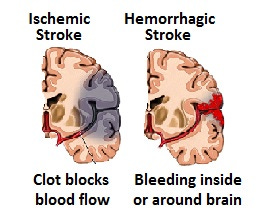
21/"Stroke After SARS-CoV-2 mRNA Vaccine: A Nationwide Registry Study"
"The cohort included 4 139 888 people, 49.8% women, and 6.7% were ≥80 years of age. During the first 28 days after an mRNA vaccine, 2104 people experienced a stroke (82% ischemic stroke, 13% intracerebral
******************************************************************************************************
👀🚨💉🧬T CELLS (CD 4 and CD 8), have their OWN cGAS STING Pathway! DNA PLASMIDs can interact w/ cGAS STING OF T CELLS, causing FUNCTION of T CELLS to be: IMPAIRED, cause T CELL DEATH, IMPEDE T CELL PROLIFERATION, hindering an effective immune response, and CANCER progression.
(multiple studies will be employed--layman's terms throughout and at the end)
1. What are T Cells and where are they?
T cells are a type of lymphocyte central to the adaptive immune system. They are found throughout the human body, primarily in lymphoid tissues and organs.
T cells mature in the thymus gland, hence the name "T cells." The thymus is located in the upper chest behind the sternum, where immature T cells from the bone marrow mature and differentiate into various types of T cells that are capable of recognizing specific antigens. Differentiate means they go through a process which changes them into the "final product", into something that is more specialized in what it does, or what we would call the final thing. We have many cells in our body that do this.
Lymph nodes are small, bean-shaped structures distributed throughout the body along lymphatic vessels, which act as filters for lymphatic fluid, where T cells along with other immune cells encounter pathogens and antigens. Lymph nodes are abundant in areas such as the neck, armpits, groin, and abdomen.
The spleen is an organ located in the upper left abdomen, which filters blood and serves as a reservoir for immune cells, including T cells.
Although T cells do not originate in the bone marrow, it is a site where hematopoietic stem cells differentiate into precursor cells that eventually migrate to the thymus for maturation into T cells.
Mucosal tissues contain T cells, like the lining of the intestines, respiratory tract, and genitourinary tract. These tissues are entry points for many pathogens, and T cells play a crucial role in defending against infections at these sites.
T cells circulate throughout the bloodstream, allowing them to quickly respond to infections and move to sites of inflammation or infection.
2. What could go wrong?
So would you think it would be a bad thing, if you did not have properly functioning T cells in these areas, or that if a bunch of them died off, the body might have a more difficult time fighting off an infection?
This is a very complicated process--we are just looking at highlights form some studies that found activation of the cGAS STING pathway, the special cGAS STING that is for T Cells, can cause them to not function properly, change them into something else, or kill them.
The first study used many methods and controls to look at what happens to T Cells when their own cGAS STING pathway is activated (we are sticking with the smoke alarm analogy for T cells).
3. Diving a bit deeper into what happens when the cGAS STING of T cells become activated (by DNA plasmid would do it):
Activation of the cGAS-STING pathway in CD4 and CD8 T cells leads to the production of antiviral cytokines such as IFNβ and IP10, which are part of the immune response against viral infections.
However, despite the beneficial antiviral response, the activation of cGAS-STING in T cells has several detrimental effects:
Increased Cell Death
There is an increase in apoptosis (programmed cell death) of T cells.
Impaired Proliferation
T cell proliferation is significantly impaired, which hinders their ability to expand and mount an effective immune response.
Metabolic Impairment
There are disturbances in cellular metabolism, including impaired glycolysis and mitochondrial function, which are critical for T cell energy production and function.
4. Heavy science time for a bit, and then swinging back around to terms that are more understandable at the end:
The impairments observed in T cells upon cGAS-STING activation involve mechanisms that are both IRF3-dependent and IRF3-independent. IRF3 is a transcription factor involved in the expression of interferons and other immune response genes. The specific pathways leading to impaired T cell function include NF-κB-mediated antiproliferative effects, ER stress-induced cell death, and IRF3/IRF7-mediated inhibition of mTORC1, among others.
5. Let's look at the cGAS STING pathway that T cells do possess (and how it has been proven):
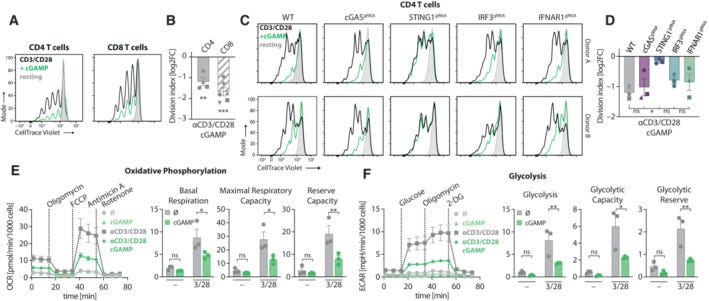
STING Activation in Human T Cells (the study)
STING, a key component of the cGAS-STING pathway, is functional in human primary T cells. When these T cells were treated with the physiological STING agonist 2′‐3′‐cGAMP (cGAMP), it induced phosphorylation of STING (pSTING) and IRF3 (pIRF3), which are critical steps in signaling through this pathway.
While cGAMP alone induced some level of STING and IRF3 phosphorylation, the activation was significantly enhanced when T cells were simultaneously activated through their T cell receptor (TCR) using αCD3/CD28 antibody-coated beads. This suggests that TCR engagement is necessary for robust activation of the cGAS-STING pathway in T cells.
The activation of STING in conjunction with TCR stimulation led to the production of antiviral factors such as IFNβ and IP10. These cytokines are critical for mounting an immune response against viral infections, indicating that T cells can indeed utilize the cGAS-STING pathway to produce these protective factors.
The study also emphasizes that the synergistic effect of STING and TCR stimulation occurs at a cell-intrinsic level, implying that T cells themselves are capable of integrating these signals to initiate an immune response.
6. How activation of this pathway in T CELLS can lead to an impaired or dysregulated response (or T cell DEATH)
Activation of STING in T cells leads to increased apoptosis (programmed cell death). This process is mediated by the transcription factor IRF3, which upregulates pro-apoptotic BH3-only proteins such as Noxa (PMAIP1). This mechanism was observed in both human and murine T cells (Gulen et al., 2017).
STING activation in T cells also inhibits their ability to proliferate. This effect is independent of IRF3 and is mediated through a distinct domain within STING's C-terminal tail (miniCTT), which disrupts the mTORC1 pathway involved in cell cycle progression (Cerboni et al., 2017).
Activation of STING alters metabolic pathways in T cells, impairing their ability to perform glycolysis and mitochondrial respiration, which are essential for energy production and cell function during activation and proliferation (Imanishi et al., 2019).
While type I interferons (IFNs) are produced upon STING activation in T cells, they do not rescue the impaired functions. Instead, they contribute to the overall dysregulation of T cell function observed in these contexts (Benoit-Lizon et al., 2022).
WHY DOES THIS MATTER?
A.) T CELLS ARE NO LONGER FUNCTIONING PROPLERY AFTER THEIR OWN cGAS STING PATHWAY HAS BEEN ENGAGED!
B.) Activation of the cGAS-STING pathway in T cells leads to increased cell death, impaired proliferation, and compromised metabolism.
This can diminish the overall effectiveness of T cell responses against viruses because T cells are critical for coordinating and executing adaptive immune responses--rendering them ineffective.
USELESS T CELLS!
C.) While the cGAS-STING pathway can trigger the production of antiviral cytokines like type I interferons, this activation also disrupts essential T cell functions, limiting the ability of T cells to proliferate and differentiate into effector cells needed to combat viral infections effectively.
D.) Long-term bad news bears
Prolonged or excessive activation of the cGAS-STING pathway in T cells could have long-term consequences for immune system health. It may lead to chronic immune dysfunction or contribute to conditions where T cell responses are dysregulated, such as autoimmune diseases or chronic viral infections, and CANCER.
E.) CANCER IMPLICATIONS:
T cells are essential for mounting an effective immune response against cancer cells.
Activation of the cGAS-STING pathway in T cells leads to functional impairment, including decreased proliferation and increased apoptosis, which reduce the ability of T cells to effectively target and eliminate cancer cells, thereby weakening the antitumor immune response.
STING activation in T cells also disrupts metabolic pathways necessary for their activation and function. This metabolic impairment can limit the energy and resources available to T cells, compromising their ability to sustain their needed defense against cancer.
In some contexts, STING activation has been associated with promoting tumor growth and immune evasion mechanisms. While STING activation can induce an initial immune response, chronic activation or dysregulation may lead to mechanisms that support tumor survival and growth, potentially through effects on the tumor microenvironment or regulatory T cells (Tregs).
******************************************************
Layman's Terms for what this means :
Imagine cGAS-STING as a smoke alarm system inside your house (representing T cells).
Its job is to detect danger signals (like DNA or damaged cells) and alert the immune system (like alerting the homeowner to a potential fire).
Impairment of T Cell Function
Normally, T cells are like firefighters that detect and eliminate cancer cells. When cGAS-STING is overly active, it's like the smoke alarm constantly going off, which exhausts the firefighters (T cells). They become less effective at finding and fighting cancer cells.
Energy Drain Time (Metabolic Dysregulation)
cGAS-STING activation in T cells disrupts their energy production, making it harder for them to stay active and fight cancer. It's like the firefighters not having enough energy to respond quickly to fires.
Mixed Signals Time (Promotion of Tumor Growth)
Sometimes, the smoke alarm (cGAS-STING) sends confusing signals. It might initially help by alerting the immune system, but if it keeps going off, it can disrupt the immune response and even help the fire (tumor) grow instead of putting it out.
The Immune System Gets WEAK
Weakened Defense: Overactive cGAS-STING in T cells weakens their ability to defend against infections and cancer. It's like having tired firefighters who can't respond effectively to emergencies.
Chronic Stress Hurts the T Cells
Continuous activation of cGAS-STING stresses out T cells, making them more prone to dysfunction. It's like the smoke alarm constantly buzzing, which wears out its batteries and makes it less reliable.
Impact on Cancer:
Mixed Bag
While cGAS-STING activation can initially boost the immune response against cancer, prolonged or excessive activation might paradoxically support tumor growth.
It's like the smoke alarm causing confusion: sometimes it helps detect the fire (cancer) early, but if it malfunctions, it might inadvertently make things worse.
Has anyone talking about how the mRNA vaccinations are impairing T cell function?
It would be really bad if there was a bunch of DNA in there, or something else in there activating the cGAS STING in T cells--and ni the thread below, you will see, that even in SMALLER AMOUNTS, LONGER pieces of DNA while HAMMER on the switch for cGAS STING.
That would be bad if it hit your cGAS STING of your T cells, would it not?
There ya go.
2/"STING agonism turns human T cells into interferon-producing cells but impedes their functionality"
ncbi.nlm.nih.gov/pmc/articles/P…
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9986811/
3/ Intrinsic antiproliferative activity of the innate sensor STING in T lymphocytes
(lymphoma?)
ncbi.nlm.nih.gov/pmc/articles/P…
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5461003/
Signalling strength determines proapoptotic functions of STING - Nature CommunicationsThe cGAS/STING signalling pathway is responsible for sensing intracellular DNA and activating downstream inflammatory genes. Here the authors show mouse primary T cells and T leukaemia are hyperrespon…https://www.nature.com/articles/s41467-017-00573-w
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10403253/
**************************************************************************
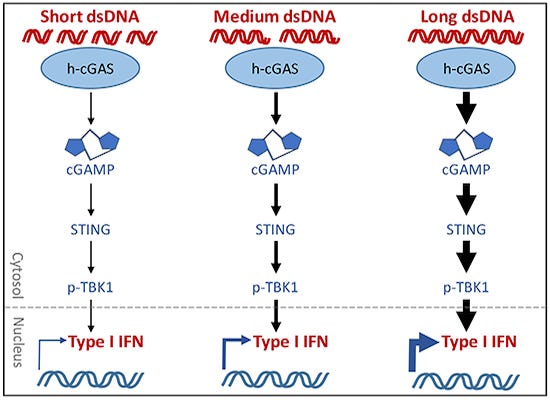
LENGTH of DNA (also type of STING--smoke detector for the body) you have (and existing mutations), is a MAJOR FACTOR in STRENGTH of IMMUNE SYSTEM (damage) Response--even at low doses. You could have LOW CONTAMINATION with LONGER PIECES of DNA and have MORE DAMAGE. STUDIES: Researchers did a study. They could have used LESS DNA pieces to track how the immune system responds when it comes to cGAS STING activation "STRENGTH" and Intensity. This does not mean that lower amounts will not do it--these are just the lowest amounts they used--we are limited in data to what the researchers have looked at. This is important to note. "cGAS is activated by DNA in a length‐dependent manner" Many people out there keep talking about dose, how much, how much, how much, how much? How much is going to hurt you inside of an LNP? I kept pushing back on this (and so have others), despite people writing articles, etc. Studies already exist. Example: The researchers found (study number one): cGAS STING pathway is activated based on DNA Length. It is LENGTH DEPENDENT when it comes to the INTENSITY of the activation (and thus, the ability to have strong response, including damage, and worse). The study demonstrates that the length of dsDNA (that is double stranded DNA, like the DNA plasmid contamination found ni the mRNA vaccines) impacts the strength of the innate immune response mediated by cGAS-STING pathway.
At high DNA concentration (1.67 μg/ml), there was no significant difference in the amount of secreted type I IFN (immune system) across the range of DNA sizes from 88 to 4,003 bp. This means even when there is a LOT more DNA plasmid contamination introduced (per vial or batch), that the immune response (well now this study does not cover the type of STING you have, see attached thread, or mutations), but just in general
At lower DNA concentrations (0.167 μg/ml and lower), a clear length-dependency was observed, where longer DNA molecules stimulated a stronger type I IFN response compared to shorter ones. That means the LESS DNA PIECES (in this study) where LONGER PIECES of DNA were present, meant that there is a stronger immune response. (this does not discount a flooding of a lot of DNA to the system and higher doses not causing issues, because that is systemic--we are talking at cellular level here). Thresholds for DNA Length The effective length thresholds for inducing a strong immune response vary with DNA concentration: At 1.67 μg/ml: No clear length-dependent response observed. At 0.167 μg/ml: Effective length threshold is around 300-500 bp for inducing a noticeable IFN response. At 0.0167 μg/ml: Effective length threshold increases to 800-2,000 bp for inducing a strong IFN response. The immune response is mediated through the cGAS-STING pathway. Long DNA molecules (e.g., 2,027 bp, 4,003 bp) are capable of stimulating IFN production at very low concentrations (0.0167 μg/ml), indicating their potent immunostimulatory capability compared to shorter DNA fragments. Experimental Confirmation This length-dependent response was confirmed across different cell types (THP-1 cells, human foreskin fibroblasts (HFFs), and primary human peripheral blood mononuclear cells (PBMCs)). Using different transfection reagents (e.g., Lipofectamine LTX, Lipofectamine RNAiMAX) and different sources of DNA (PCR-derived, plasmid backbone-derived restriction fragments), consistent length-dependent responses were observed. :Downstream mechanistic analyses revealed that the length-dependency occurs at the level of cGAS activation and cGAMP production, involving phosphorylation of TBK1, dimerization and phosphorylation of STING, and cGAMP synthesis. Longer DNA molecules are more potent stimulators of the innate immune system, particularly at lower concentrations, indicating a threshold effect where longer DNA molecules can trigger robust immune responses even at low concentrations, compared to shorter DNA fragments. Please throw out the notion that less DNA contamination is going to be OK.
**************************************************************************
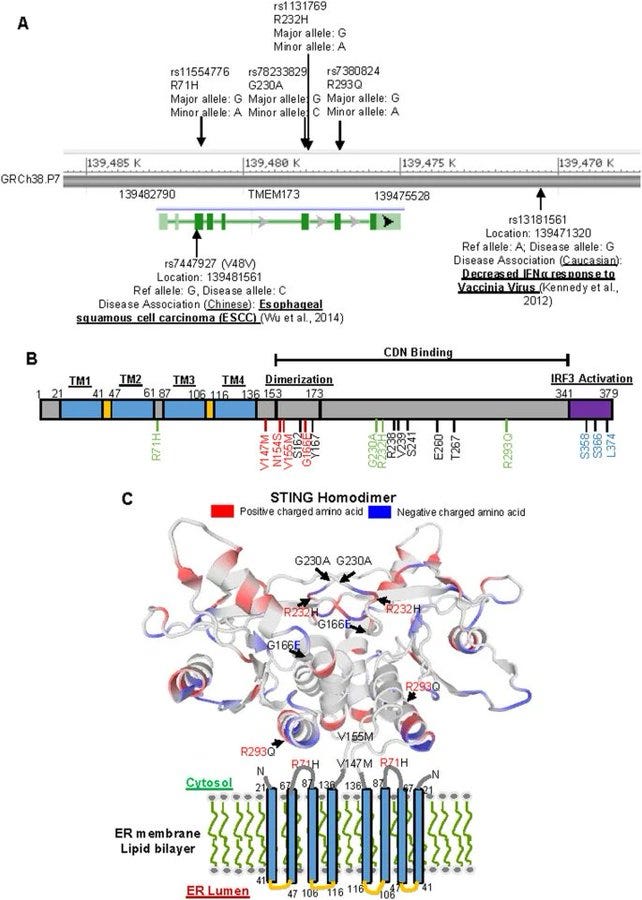
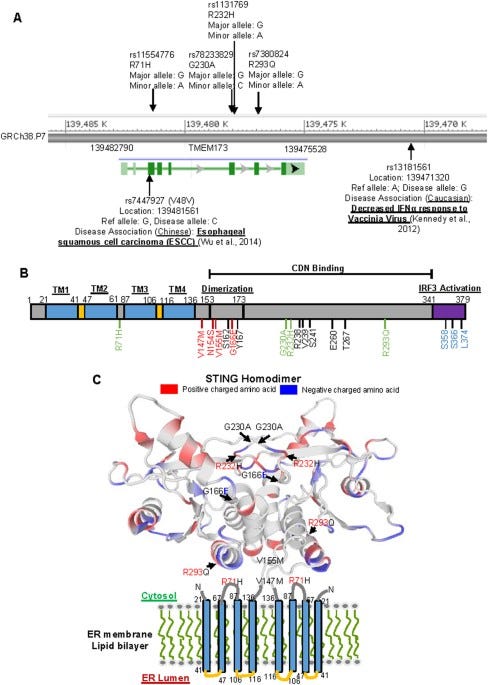
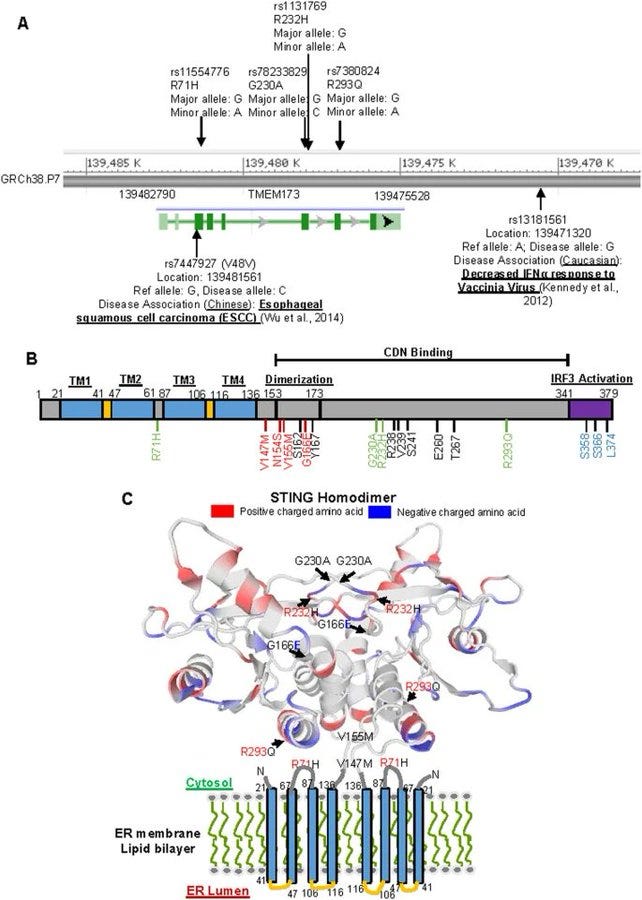
cGAS STING pathway is implicated in most vaccine injuries: STING Type I (R232) Most common form found in populations of African descent. STING Type II (H232) Prevalence: Predominant in East Asian populations. STING Type III (R71H-G230A-R293Q) Common in European and Middle Eastern populations. STING Type IV (R293Q) Primarily found in Indigenous populations of the Americas. This pathway is the primary cause of most of the vaccine injuries we are seeing, outside of clots, although it can be part of that cascade. The protein I have been posting about, is not spike, that is obvious to any scientist. This is the STING protein, that is part of the cGAS STING pathway. In the area in blue, the sting protein is featured, and below it, all diseases which are impacted by the hyperactivation, or dysregulation of it, through exposure to DNA plasmid pieces that DO NOT BELONG IN ANY AMO0UNT IN THE CELLS IN OUR BODY and will cause activation of this pathway and immune system attack, the spike protein, or bacteria. STING Type III (R71H-G230A-R293Q) Common in European and Middle Eastern populations is important because this variation of STING has the highest prevalence of AUTOIMMUNE DYSFUNCTION AND AGRESSIVE CANCER RATES.
*****************************************************************************************
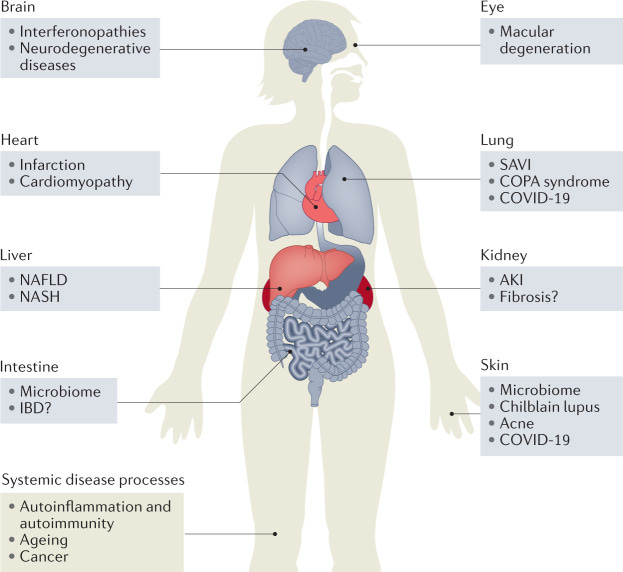
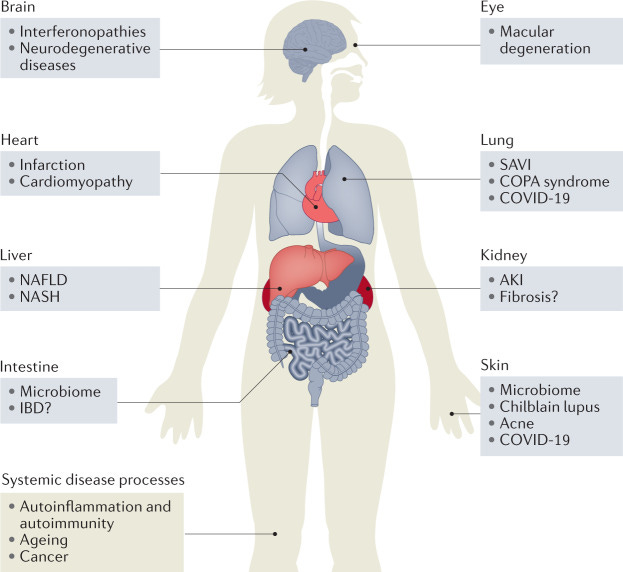
COVID 19 "VACCINES" CAUSING MULTI SYSTEM ORGAN DAMAGE AND FAILURE through (hyper) ACTIVATION/DYSREGULATION of cGAS STING pathway
by interacting with DNA PLASMID contamination, SPIKE PROTEIN, and BACTERIAL CONTAMINATION.
(variants of STING (SNP) and mutation of STING)
2/ This could occur over the course of 24-72 hours, or over weeks, or TWO MONTHS.
The person receives a mRNA COVID VACCINE and is exposed to DNA plasmid pieces encapsulated within lipid nanoparticles, and there is expression of spike protein, and possible bacteria (LPS).
3/ The LNP facilitate the entry of the DNA plasmids into cells, where they activate the cGAS-STING pathway.
Initial symptoms might include mild flu-like symptoms such as fever, fatigue, and malaise due to the initial immune response and cytokine release.
4/ (READ THIS FOR FULL ACTIVATION PATHWAY SANS THE STING variants/mutations):
christiegrace.substack.com/p/cgas-sting-p…
cGAS STING Pathway activation by DNA Plasmid Contamination, SPIKE, and LPS in modRNA "vaccines": AIDP, Myocarditis, Stroke, Aortic Dissection, and More: Overview, and Biopsy Methods for Detection.SUPER cGAS STING SUBSTACK: Detection methods for scientists/pathologists towards the endhttps://christiegrace.substack.com/p/cgas-sting-pathway-activation-by
5/ The continuous presence of DNA plasmid pieces/spike maintains the hyperactivation of the cGAS-STING pathway.
STING can become dysregulated for a few reasons. FLOOD of DNA plasmids and over expression of SPIKE.
Person can have a variant of STING that sets them up for this.
6/
There are four variants of STING based on RACE:
STING Type III (R71H-G230A-R293Q) Common in European and Middle Eastern populations.
STINGIII has the highest prevalence of autoimmune disease, cancer, stroke, myocarditis, AAD, etc.
7/ there are also mutations within these variants (the variants of STING have a SNP)
Unroll available on Thread Reader
8/ these variants of STING (if you are WHITE or middle eastern) can set you up to be more susceptible to cGAS STING dysregulation.
And what can happen, is cGAS STING can enter a positive feedback loop.
9/ Positive Feedback Loop: The release of self-DNA and continued activation of the cGAS-STING pathway create a positive feedback loop, amplifying the immune response and sustaining inflammation because cells that are getting damaged because the immune system is attacking
10/ release their own DNA, which activates cGAS AGAIN, activating a feedback loop. Even if you eradicate the DNA plasmid or spike, if this pathway become engaged in a LOOP, that's it, it is now attacking self.
11/ (if slow moving organ failure--fast moving over 3 days would go rapidly to last conditions listed): Symptoms could escalate to more severe fatigue, muscle and joint pain, and potentially signs of systemic inflammation such as rashes or swelling.
12/ arly signs of organ involvement may begin, such as mild liver enzyme elevations (liver inflammation) or proteinuria (kidney involvement).
Persistent activation leads to a cytokine storm, with widespread release of pro-inflammatory cytokines.
13/ severe symptoms, including persistent high fevers, significant fatigue, and weight loss.
Clinical signs of organ dysfunction become evident, such as jaundice (liver failure), shortness of breath (lung involvement), and swelling or edema (kidney dysfunction).
14/ the person's condition deteriorates significantly as multiple organs are affected by the ongoing inflammatory damage by the hyperactivation of the cGAS STING pathway.
Severe symptoms might include confusion or altered mental status (brain involvement),
15/ severe respiratory distress, and heart failure symptoms such as chest pain or arrhythmias.
e cumulative damage leads to the failure of critical organs, and despite medical intervention, the body cannot sustain vital functions.
16/ 🚨💉 Fluid retention around the eyes leads to periorbital edema, which manifests as puffiness and dark circles due to the thin skin and visible blood vessels under the eyes.
Accumulation of waste products in the blood will also do this. @Answers4Sean
17/ 🚨💉NONE OF THIS IS CAUSED BY DNA INTENGRATION OR SV40.
@Answers4Sean
Teenage boys generally have a higher BMR due to greater muscle mass and physical activity levels, which can exacerbate the metabolic demands during systemic inflammation and organ failure.
18/ During puberty, boys have higher levels of androgens, amplifying immune responses and inflammatory processes. Androgens enhance the production of certain cytokines, intensifying the inflammatory response.
THIS also explains why YOUNG MEN GET MYOCARDITIS @SenatorRennick
19/ 🚨💉 immune system in adolescents is still maturing, potentially leading to an exaggerated inflammatory response when the cGAS-STING pathway is chronically activated.
20/ Multisystem Inflammation and Organ Dysfunction After BNT162b2 Messenger RNA Coronavirus Disease 2019 Vaccination
21/ 🚨💉 A 14-year-old Japanese girl died unexpectedly 2 days after the BNT1262b2 mRNA COVID-19 vaccine. Autopsy findings showed congestive edema of the lungs, T-cell lymphocytic and macrophage infiltration in the lungs, pericardium, and myocardium of the
22/ left atria and left ventricle, liver, kidneys, stomach, duodenum, bladder, and diaphragm
A case of fatal multi-organ inflammation following COVID-19 vaccination - PubMedA 14-year-old Japanese girl died unexpectedly 2 days after receiving the third dose of the BNT1262b2 mRNA COVID-19 vaccine. Autopsy findings showed congestive edema of the lungs, T-cell lymphocytic an…https://pubmed.ncbi.nlm.nih.gov/36990036/
23/
Role of the cGAS–STING pathway in systemic and organ-specific diseases
.
Case Report: Severe Rhabdomyolysis and Multiorgan Failure After ChAdOx1 nCoV-19 Vaccinationchristiegrace.substack.com/p/cgas-sting-p…
ncbi.nlm.nih.gov/pmc/articles/P…
ncbi.nlm.nih.gov/pmc/articles/P…
cGAS STING Pathway activation by DNA Plasmid Contamination, SPIKE, and LPS in modRNA "vaccines": AIDP, Myocarditis, Stroke, Aortic Dissection, and More: Overview, and Biopsy Methods for Detection.SUPER cGAS STING SUBSTACK: Detection methods for scientists/pathologists towards the endhttps://christiegrace.substack.com/p/cgas-sting-pathway-activation-by
https://www.sciencedirect.com/science/article/pii/S2590124924000038
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9214686/
24/
frontiersin.org/journals/immun…
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10027302/
Frontiers | Case Report: Severe Rhabdomyolysis and Multiorgan Failure After ChAdOx1 nCoV-19 VaccinationBackgroundSevere skeletal muscle damage has been recently reported in patients with SARS-CoV-2 infection and as a rare vaccination complication.Case summaryO...https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2022.845496/full
************************************************************************************
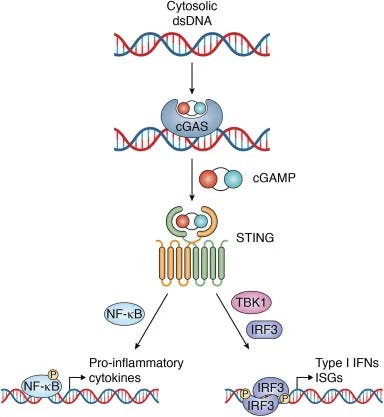
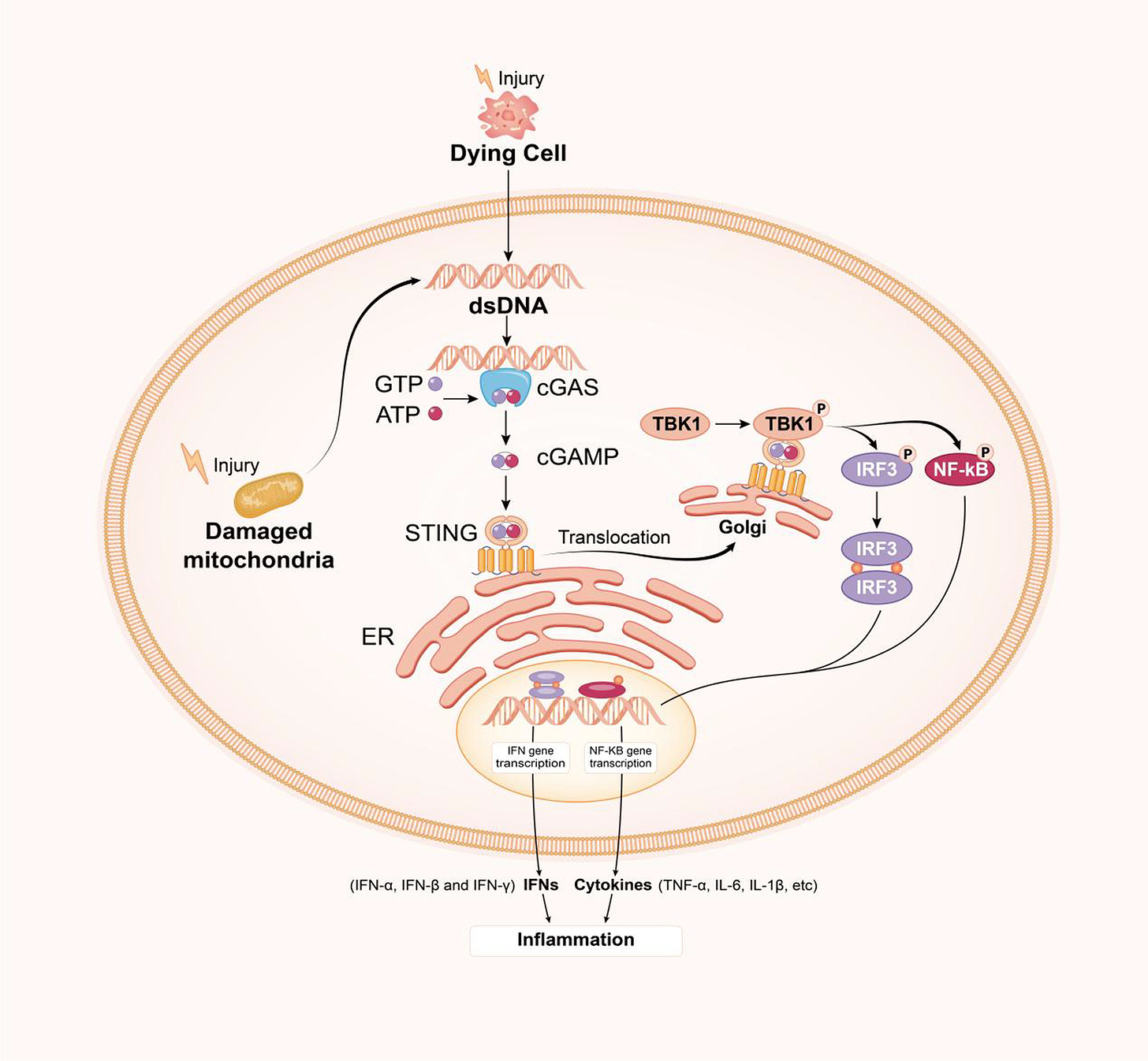
CANCER: "COMBINED" FEEDBACK LOOP of cGAS STING AND APOBEC: DNA plasmid in LNP +DS RNA triggers cGAS STING AND induction of DNA deaminase APOBEC3A + nuclear DNA damage: Dysregulation/ positive feedback loops of cGAS STING meets APOBEC (a DIFFERENT MULTI-HIT mechanism): This is obviously, not meant for X (twitter) but here we are. Hard science time. A. First read substack below for cGAS STING activation--massive stack: on the RNA/LNP combo. Here are some highlights from that: The cGAS-STING pathway is a critical component of the innate immune system. It serves as sensor for cytosolic DNA and orchestrating the cellular response to various microbial infections, cellular stress, and DNA damage. You could think of it like a smoke detector sensing smoke, and then activating an alarm system, that responds to danger, but worse. cGAS is a cytosolic DNA sensor that recognizes double-stranded DNA (dsDNA) and double stranded RNA (and spike)derived from pathogens, damaged cells, or cellular debris. (I am not going to ncontinue to type double stranded RNA for activation of cGAS or spike to save space, time, and to avoid constant redundancy) Upon DNA binding, cGAS catalyzes the synthesis of cyclic GMP-AMP (cGAMP) from ATP and GTP. STING (Stimulator of Interferon Genes): STING is an endoplasmic reticulum (ER)-resident protein that serves as a signaling adaptor downstream of cGAS. Recognition of Pathogen DNA: cGAS detects cytosolic DNA derived from bacteria, viruses, or other pathogens. Pathogen DNA may be released during infection, replication, or cell lysis. DNA that is exogenous DOES include what is called ODN—the pieces of plasmid DNA that are in the current modRNA “vaccines” will also activate this pathway. Spike protein will also activate, and there are recent studies showing that DS DNA will do it too. Upon binding to cGAMP or other cyclic dinucleotides (CDNs), STING undergoes conformational changes and translocates from the ER to perinuclear puncta, where it recruits downstream signaling effectors. Infammatory conditions, such as autoimmune diseases, cancer, or tissue injury, can lead to the release of self-DNA or DAMPs (damage-associated molecular patterns), activating the cGAS-STING pathway in infiltrating immune cells or stromal cells. Activation of the cGAS-STING pathway induces the expression of type I interferons and other antiviral effectors, leading to the inhibition of viral replication, clearance of infected cells, and activation of adaptive immune responses. Dysregulation of the cGAS-STING pathway has been implicated in various autoimmune diseases, including systemic lupus erythematosus (SLE), Aicardi-Goutières syndrome (AGS), and type I interferonopathies, where aberrant activation of the pathway leads to the production of autoantibodies, tissue inflammation, and organ damage. Inflammatory processes can lead to cell death or damage, resulting in the release of self-DNA from damaged or dying cells. This self-DNA can further activate the cGAS-STING pathway in neighboring cells or immune cells, perpetuating the immune response. Positive Feedback Loop: The release of self-DNA and continued activation of the cGAS-STING pathway create a positive feedback loop, amplifying the immune response and sustaining inflammation. This amplification mechanism can contribute to chronic inflammation and autoimmune diseases if dysregulated, including MS, AIDP, Sjögren's Syndrome, Myocarditis, and more. The FEEDBACK LOOP: HOW the cGAS STING PATHWAY IS MAINTAINED IN THE “ON” POSITION—CONSTANT INFLAMMATION, AUTOIMMUNE ATTACK, AND WORSENING OF CONDITIONS--including CANCER EVEN IF THERE IS NO LONGER A PRESENCE OF PLASMID DNA, Double Stranded RNA, SPIKE PROTEIN, OR LPS: Positive Feedback Loops: Upon activation, the cGAS-STING pathway triggers the production of type I interferons (IFNs) and other inflammatory cytokines, which in turn promote the expression of interferon-stimulated genes (ISGs). Some ISGs encode proteins that directly amplify the cGAS-STING signaling pathway or modulate its activity, creating a positive feedback loop that reinforces immune responses. These proteins may include factors involved in signal transduction, transcriptional regulation, or post-translational modifications. Inflammatory Signaling Cascades: In addition to type I IFNs, other pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) can activate downstream signaling pathways, including NF-κB and MAPK pathways. These signaling cascades further enhance the expression of inflammatory mediators, perpetuating inflammation and immune cell activation. Chronic Tissue Damage and Inflammation: Prolonged activation of the cGAS-STING pathway and sustained immune responses can lead to chronic tissue damage and inflammation. Cellular stress, DNA damage, and mitochondrial dysfunction may exacerbate cGAS-STING activation, creating a cycle of tissue injury and immune activation. Damaged tissues release danger-associated molecular patterns (DAMPs), including self-DNA, ATP, and HMGB1, which further stimulate innate immune responses and maintain inflammation. Autoimmune Responses: In autoimmune diseases, the cGAS-STING pathway may become dysregulated, leading to the recognition of self-DNA as foreign and the generation of autoantibodies against self-antigens. Autoantibodies, particularly those targeting nuclear antigens, can form immune complexes that activate complement cascades and recruit immune cells, perpetuating tissue inflammation and injury. Epigenetic Regulation: Epigenetic modifications, including DNA methylation, histone modifications, and non-coding RNA regulation, can modulate the activity of genes involved in the cGAS-STING pathway and immune responses. Epigenetic changes induced by chronic inflammation or cellular stress can contribute to the persistence of immune activation and the development of autoimmune phenotypes. Positive Feedback Loops: Upon activation by type I interferons (IFNs), ISGs are upregulated, many of which encode proteins involved in amplifying the cGAS-STING signaling pathway. For example, IFN-induced proteins such as IFIT1, IFIT3, and MX1 can directly interact with components of the pathway to enhance signaling or promote downstream effector functions. Amplification of Signaling Pathways: Some ISGs may modulate the activity of key signaling molecules involved in the cGAS-STING pathway, such as TBK1 (TANK-binding kinase 1) and IRF3 (interferon regulatory factor 3), leading to the amplification of downstream signaling events and the sustained production of inflammatory mediators. Inflammatory Signaling Cascades: NF-κB Pathway: Activation of NF-κB signaling by inflammatory cytokines such as TNF-α and IL-1β can synergize with the cGAS-STING pathway to enhance the expression of pro-inflammatory genes. NF-κB target genes include cytokines, chemokines, and adhesion molecules that recruit immune cells to sites of inflammation and promote tissue damage. MAPK Pathway: Mitogen-activated protein kinase (MAPK) signaling pathways, including the ERK, JNK, and p38 MAPK pathways, can be activated by inflammatory stimuli and contribute to the regulation of gene expression, cell proliferation, and immune responses. MAPK activation downstream of cGAS-STING signaling may further amplify inflammatory signaling cascades and immune cell activation. Chronic Tissue Damage and Inflammation: Cellular Stress and DNA Damage: Persistent activation of the cGAS-STING pathway and sustained immune responses can lead to cellular stress, DNA damage, and mitochondrial dysfunction. Accumulation of damaged cellular components and reactive oxygen species (ROS) contributes to tissue injury and inflammation, perpetuating immune activation and exacerbating tissue damage. Loss of Self-Tolerance: Dysregulated activation of the cGAS-STING pathway and prolonged exposure to self-DNA can lead to the breakdown of immune tolerance and the generation of autoreactive immune responses. Autoantibodies targeting self-antigens, such as nuclear antigens in systemic lupus erythematosus (SLE), form immune complexes that drive tissue inflammation and contribute to autoimmune pathology. Epigenetic Regulation: DNA Methylation and Histone Modifications: Epigenetic modifications can regulate the expression of genes involved in the cGAS-STING pathway and immune responses. Changes in DNA methylation patterns and histone modifications may alter the accessibility of gene promoters, enhancers, and regulatory elements, influencing the transcriptional activity of key immune genes. Cell Death: Activation of the cGAS-STING pathway can induce various forms of programmed cell death, including apoptosis, pyroptosis, and necroptosis. These cell death pathways help to eliminate infected or damaged cells, contributing to host defense and tissue homeostasis. DNA Damage Response: The cGAS-STING pathway can influence the DNA damage response by regulating DNA repair mechanisms and genomic stability. Activation of STING has been linked to the induction of DNA damage repair pathways, such as homologous recombination and non-homologous end joining. Senescence: Activation of the cGAS-STING pathway has been implicated in cellular senescence, a state of irreversible growth arrest associated with aging and various pathological conditions. Senescent cells exhibit increased expression of cGAS and STING, suggesting a potential role for the pathway in driving senescence-associated inflammation and tissue dysfunction. Immune Cell Activation: The cGAS-STING pathway modulates the activation and function of immune cells, including dendritic cells, macrophages, and T cells. Activation of STING in dendritic cells enhances antigen presentation and T cell priming, promoting adaptive immune responses against pathogens or tumor cells, which actually can take a turn for the worst (more in a bit on that). Cancer: The cGAS-STING pathway plays a critical role in the detection of tumor-derived DNA and the activation of anti-tumor immune responses. If this pathway gets dysregulated, you are in trouble. Inflammatory cytokines and reactive oxygen species (ROS) generated during chronic inflammation can damage cellular DNA. This damage can lead to the formation of more cytosolic DNA fragments, perpetuating the activation of the cGAS-STING pathway. Chronic inflammation and the resulting DNA damage can interfere with normal DNA repair processes. Error-prone repair mechanisms may become more prevalent, increasing the likelihood of mutations and genomic instability. DNA damage creates more cytosolic DNA fragments, which further activate cGAS. This leads to more production of cGAMP and subsequent STING activation, maintaining a cycle of inflammation and DNA damage. Feedback Loops in cGAS-STING Pathway Inflammation-Induced DNA Damage: Inflammatory cytokines, such as TNF-α and IL-6, contribute to cellular stress and DNA damage. ROS production during chronic inflammation also damages DNA. DNA damage can result in chromosomal aberrations and the formation of micronuclei. Micronuclei rupture, releasing DNA into the cytosol, which is detected by cGAS. The presence of cytosolic DNA continuously activates the cGAS-STING pathway. This leads to sustained production of inflammatory mediators, creating a persistent inflammatory state. Chronic inflammatory signaling can downregulate effective DNA repair pathways. The cellular environment becomes more conducive to accumulating mutations due to error-prone repair mechanisms. B. Now let's add in APOBEC to the equation, when talking about DNA plasmids (and spike perhaps) AND double stranded RNA--both from OUR cells (mitochondria) AND if it were present in the lipid nanoparticles inside the COVID vaccines--you have TWO sources of it now) and what that would look like if cGAS STING got dysregulated, and how that ties into APOBEC mutations: APOBEC (Apolipoprotein B mRNA Editing Enzyme, Catalytic Polypeptide-like) enzymes are a family of cytidine deaminases involved in RNA editing and innate immune responses. Mutations or overexpression of APOBEC enzymes, especially APOBEC3, can lead to genomic instability through their off-target activity on single-stranded DNA (ssDNA), which often occurs during DNA replication or repair. Here's how APOBEC mutations and activity relate to the cGAS-STING Off-Target Mutagenesis: APOBEC enzymes typically target viral genomes to induce mutations that inhibit viral replication. However, when these enzymes act on cellular ssDNA, they introduce mutations by deaminating cytosine to uracil. This off-target activity can lead to a high mutation burden, causing genomic instability and contributing to cancer development. The uracil bases introduced by APOBEC activity are recognized as lesions by the DNA repair machinery, leading to base excision repair (BER). However, if repair is faulty or overwhelmed, this can result in double-strand breaks (DSBs), further increasing genomic instability. Activation by DNA Damage: The cyclic GMP-AMP synthase (cGAS) senses cytosolic DNA, which can result from cellular stress, DNA damage, or viral infection. Upon recognizing DNA, cGAS synthesizes cyclic GMP-AMP (cGAMP), a second messenger that activates the Stimulator of Interferon Genes (STING) pathway. Activated STING triggers a signaling cascade leading to the production of type I interferons and other inflammatory cytokines, promoting an antiviral state and recruiting immune cells to the site of infection or damage. So now, DNA damage and genomic instability caused by APOBEC enzymes can lead to the presence of cytosolic DNA fragments, which can activate the cGAS-STING pathway. This links APOBEC-induced mutagenesis and genomic instability with the activation of an innate immune response--which is activation of cGAS STING by DNA plasmids, DS RNA, spike, etc. (FYI, this could be happening without any vaccines). The Tumor Microenvironment: Chronic activation of the cGAS-STING pathway due to persistent DNA damage and genomic instability can contribute to an inflammatory tumor microenvironment. This can influence cancer progression and the immune response to tumors. Summary: APOBEC-induced genomic instability can activate the cGAS-STING pathway through the generation of cytosolic DNA, linking mutagenesis and DNA damage to innate immune activation. However, cGAS STING activation can generate APOBEC genomic instability, introducing the chiekn versus the egg scenario, or, is it option THREE--it's happening in tandem, and at intersecting points. This relationship is significant in the context of cancer development and the tumor immune microenvironment. cGAS STING meets APOBEC. But if we were to proceed in a more linear fashion: DNA Damage and Cytosolic DNA: Various factors such as oxidative stress, radiation, chemotherapy, or replication stress can cause DNA damage, resulting in the formation of double-strand breaks (DSBs). During the repair process, fragments of DNA can end up in the cytosol. The presence of cytosolic DNA activates cGAS. The other thigns that can activate cGAS, the smoke detector for the human body, are pices of plasmid DNA contamination, double stranded RNA (hope this was not recently found in COVID vaccines), double stranded RNA from our own body (mitochondria), or other source. Spike protein will also activate cGAS, and bacteria, so now the smoke detector is activated, and it triggers the immune response, like water raining down on a fire, but the water is going to do damage, to our cells, our house. So once again, be it double stranded RNA, DNA plasmids, spike, or bacteria, we now have cGAS Activation: cGAS binds to cytosolic DNA and catalyzes the synthesis of cyclic GMP-AMP (cGAMP). STING Activation: cGAMP binds to and activates the STING protein located on the endoplasmic reticulum membrane. Activated STING initiates a signaling cascade involving TBK1 and IRF3, leading to the production of type I interferons (IFNs) and other inflammatory cytokines. This is important in APOBEC in tandem with cGAS STING, like chocolate and peanut butter. Genomic Instability and APOBEC Activity through cGAS STING ACTIVATION: The production of type I IFNs and cytokines leads to an inflammatory response, attracting immune cells to the site of damage. Chronic inflammation and immune responses can induce further DNA damage, OUR DNA, in OUR CELLS, and these include MITOCHONDRIA, which are going to release their own dsRNA , because "Mitochondrial double-stranded RNA triggers induction of the antiviral DNA deaminase APOBEC3A and nuclear DNA damage" --exacerbating genomic instability. Persistent DNA damage and repair attempts increase replication stress. During this process, single-stranded DNA (ssDNA) regions become more frequent, serving as substrates for APOBEC enzymes. ALSO, if you already have double stranded RNA within an LNP, you are ALSO going to have these acting as a substrate for APOBEC enzymes. And now, not only is cGAS STING activated, so is APOBEC. APOBEC Activation: The innate immune response, particularly through interferon signaling, can upregulate APOBEC enzymes as part of the antiviral response. APOBEC enzymes deaminate cytosine to uracil in ssDNA, introducing mutations. MORE mutations. The cGAS-STING pathway, once activated, can contribute to genomic instability and potentially influence APOBEC activity, creating a feedback loop that drives cancer progression and impacts the tumor microenvironment. Here’s a detailed sequence of events illustrating this process: Activation of the cGAS-STING Pathway DNA Damage and Cytosolic DNA:Various factors such as oxidative stress, radiation, chemotherapy, or replication stress can cause DNA damage, resulting in the formation of double-strand breaks (DSBs). During the repair process, fragments of DNA can end up in the cytosol. The presence of cytosolic DNA activates cGAS. cGAS Activation:cGAS binds to cytosolic DNA and catalyzes the synthesis of cyclic GMP-AMP (cGAMP). STING Activation:cGAMP binds to and activates the STING protein located on the endoplasmic reticulum membrane. Activated STING initiates a signaling cascade involving TBK1 and IRF3, leading to the production of type I interferons (IFNs) and other inflammatory cytokines. Genomic Instability and APOBEC Activity Inflammatory Response:The production of type I IFNs and cytokines leads to an inflammatory response, attracting immune cells to the site of damage. Chronic inflammation and immune responses can induce further DNA damage, exacerbating genomic instability. DNA Repair and Replication Stress:Persistent DNA damage and repair attempts increase replication stress. During this process, single-stranded DNA (ssDNA) regions become more frequent, serving as substrates for APOBEC enzymes. APOBEC Activation:The innate immune response, particularly through interferon signaling, can upregulate APOBEC enzymes as part of the antiviral response. APOBEC enzymes deaminate cytosine to uracil in ssDNA, introducing mutations. Feedback Loop and Cancer Progression APOBEC-Induced Mutations:APOBEC-induced mutations can lead to a high mutational burden, contributing to genomic instability and creating mutations that can drive cancer progression. Some of these mutations may inactivate tumor suppressor genes or activate oncogenes, promoting cancer development and evolution. Further Activation of cGAS-STING:As genomic instability persists, more DNA fragments may accumulate in the cytosol, perpetuating the activation of the cGAS-STING pathway. Impact on Tumor Microenvironment:The cGAS-STING pathway’s activation leads to chronic inflammation within the tumor microenvironment, which can support tumor growth and immune evasion. Inflammatory cytokines can create a microenvironment that is more conducive to cancer progression by promoting angiogenesis, metastasis, and suppressing anti-tumor immune responses. Summary Initial DNA damage activates the cGAS-STING pathway, causing inflammation and immune responses. Chronic activation of this pathway leads to further DNA damage and genomic instability. Genomic instability provides substrates for APOBEC enzymes, which introduce mutations. APOBEC-induced mutations further increase genomic instability and contribute to cancer progression. Persistent DNA damage results in continued activation of the cGAS-STING pathway, creating a feedback loop. The tumor microenvironment becomes inflamed and supportive of cancer progression due to chronic activation of the cGAS-STING pathway and APOBEC loop combined. C. NOW Let's delve deeper into the detailed sequence of events illustrating how the cGAS-STING pathway can contribute to genomic instability and influence APOBEC activity, creating a feedback loop that drives cancer progression and impacts the tumor microenvironment. We’ll start from the introduction of plasmid DNA and DOUBLE STRANDED RNA (and spike) into cells using lipid nanoparticles and proceed through the sequence of events.
COVID 19 "VACCINES" CAUSING MULTI SYSTEM ORGAN DAMAGE AND FAILURE through (hyper) ACTIVATION/DYSREGULATION of cGAS STING pathway
by interacting with DNA PLASMID contamination, SPIKE PROTEIN, and BACTERIAL CONTAMINATION.
(variants of STING (SNP) and mutation of STING)
2/ This could occur over the course of 24-72 hours, or over weeks, or TWO MONTHS.
The person receives a mRNA COVID VACCINE and is exposed to DNA plasmid pieces encapsulated within lipid nanoparticles, and there is expression of spike protein, and possible bacteria (LPS).
3/ The LNP facilitate the entry of the DNA plasmids into cells, where they activate the cGAS-STING pathway.
Initial symptoms might include mild flu-like symptoms such as fever, fatigue, and malaise due to the initial immune response and cytokine release.
4/ (READ THIS FOR FULL ACTIVATION PATHWAY SANS THE STING variants/mutations):
christiegrace.substack.com/p/cgas-sting-p…
cGAS STING Pathway activation by DNA Plasmid Contamination, SPIKE, and LPS in modRNA "vaccines": AIDP, Myocarditis, Stroke, Aortic Dissection, and More: Overview, and Biopsy Methods for Detection.SUPER cGAS STING SUBSTACK: Detection methods for scientists/pathologists towards the endhttps://christiegrace.substack.com/p/cgas-sting-pathway-activation-by
5/ The continuous presence of DNA plasmid pieces/spike maintains the hyperactivation of the cGAS-STING pathway.
STING can become dysregulated for a few reasons. FLOOD of DNA plasmids and over expression of SPIKE.
Person can have a variant of STING that sets them up for this.
6/
There are four variants of STING based on RACE:
STING Type III (R71H-G230A-R293Q) Common in European and Middle Eastern populations.
STINGIII has the highest prevalence of autoimmune disease, cancer, stroke, myocarditis, AAD, etc.
7/ there are also mutations within these variants (the variants of STING have a SNP)
Unroll available on Thread Reader
8/ these variants of STING (if you are WHITE or middle eastern) can set you up to be more susceptible to cGAS STING dysregulation.
And what can happen, is cGAS STING can enter a positive feedback loop.
9/ Positive Feedback Loop: The release of self-DNA and continued activation of the cGAS-STING pathway create a positive feedback loop, amplifying the immune response and sustaining inflammation because cells that are getting damaged because the immune system is attacking
10/ release their own DNA, which activates cGAS AGAIN, activating a feedback loop. Even if you eradicate the DNA plasmid or spike, if this pathway become engaged in a LOOP, that's it, it is now attacking self.
11/ (if slow moving organ failure--fast moving over 3 days would go rapidly to last conditions listed): Symptoms could escalate to more severe fatigue, muscle and joint pain, and potentially signs of systemic inflammation such as rashes or swelling.
12/ arly signs of organ involvement may begin, such as mild liver enzyme elevations (liver inflammation) or proteinuria (kidney involvement).
Persistent activation leads to a cytokine storm, with widespread release of pro-inflammatory cytokines.
13/ severe symptoms, including persistent high fevers, significant fatigue, and weight loss.
Clinical signs of organ dysfunction become evident, such as jaundice (liver failure), shortness of breath (lung involvement), and swelling or edema (kidney dysfunction).
14/ the person's condition deteriorates significantly as multiple organs are affected by the ongoing inflammatory damage by the hyperactivation of the cGAS STING pathway.
Severe symptoms might include confusion or altered mental status (brain involvement),
15/ severe respiratory distress, and heart failure symptoms such as chest pain or arrhythmias.
e cumulative damage leads to the failure of critical organs, and despite medical intervention, the body cannot sustain vital functions.
16/ 🚨💉 Fluid retention around the eyes leads to periorbital edema, which manifests as puffiness and dark circles due to the thin skin and visible blood vessels under the eyes.
Accumulation of waste products in the blood will also do this. @Answers4Sean
17/ 🚨💉NONE OF THIS IS CAUSED BY DNA INTENGRATION OR SV40.
@Answers4Sean
Teenage boys generally have a higher BMR due to greater muscle mass and physical activity levels, which can exacerbate the metabolic demands during systemic inflammation and organ failure.
18/ During puberty, boys have higher levels of androgens, amplifying immune responses and inflammatory processes. Androgens enhance the production of certain cytokines, intensifying the inflammatory response.
THIS also explains why YOUNG MEN GET MYOCARDITIS @SenatorRennick
19/ 🚨💉 immune system in adolescents is still maturing, potentially leading to an exaggerated inflammatory response when the cGAS-STING pathway is chronically activated.
20/ Multisystem Inflammation and Organ Dysfunction After BNT162b2 Messenger RNA Coronavirus Disease 2019 Vaccination
21/ 🚨💉 A 14-year-old Japanese girl died unexpectedly 2 days after the BNT1262b2 mRNA COVID-19 vaccine. Autopsy findings showed congestive edema of the lungs, T-cell lymphocytic and macrophage infiltration in the lungs, pericardium, and myocardium of the
22/ left atria and left ventricle, liver, kidneys, stomach, duodenum, bladder, and diaphragm
A case of fatal multi-organ inflammation following COVID-19 vaccination - PubMedA 14-year-old Japanese girl died unexpectedly 2 days after receiving the third dose of the BNT1262b2 mRNA COVID-19 vaccine. Autopsy findings showed congestive edema of the lungs, T-cell lymphocytic an…https://pubmed.ncbi.nlm.nih.gov/36990036/
23/
Role of the cGAS–STING pathway in systemic and organ-specific diseases
.
Case Report: Severe Rhabdomyolysis and Multiorgan Failure After ChAdOx1 nCoV-19 Vaccinationchristiegrace.substack.com/p/cgas-sting-p…
ncbi.nlm.nih.gov/pmc/articles/P…
ncbi.nlm.nih.gov/pmc/articles/P…
cGAS STING Pathway activation by DNA Plasmid Contamination, SPIKE, and LPS in modRNA "vaccines": AIDP, Myocarditis, Stroke, Aortic Dissection, and More: Overview, and Biopsy Methods for Detection.SUPER cGAS STING SUBSTACK: Detection methods for scientists/pathologists towards the endhttps://christiegrace.substack.com/p/cgas-sting-pathway-activation-by
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9214686/
https://www.sciencedirect.com/science/article/pii/S2590124924000038
24/
frontiersin.org/journals/immun…
Frontiers | Case Report: Severe Rhabdomyolysis and Multiorgan Failure After ChAdOx1 nCoV-19 VaccinationBackgroundSevere skeletal muscle damage has been recently reported in patients with SARS-CoV-2 infection and as a rare vaccination complication.Case summaryO...https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2022.845496/full
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10027302/
****************************************************************************************
Hypothetical: Rapid Onset of Paralysis after COVID 19 Vaccination: potential cause: cGAS-STING "hyper" Activation a WITH INCREASED activation due to physioolgical differences in teenager compared to adult.
This thread will discuss upsetting scenario.
(Heavy science)
2/ Emerging role of STING signalling in CNS injury: inflammation, autophagy, necroptosis, ferroptosis and pyroptosis
jneuroinflammation.biomedcentral.com/articles/10.11…
Emerging role of STING signalling in CNS injury: inflammation, autophagy, necroptosis, ferroptosis and pyroptosis - Journal of NeuroinflammationStimulator of interferons genes (STING), which is crucial for the secretion of type I interferons and proinflammatory cytokines in response to cytosolic nucleic acids, plays a key role in the innate i…https://jneuroinflammation.biomedcentral.com/articles/10.1186/s12974-022-02602-y
3/ Scenario: teen is given covid vaccine mRNA based, may or may not have NDA plasmid contamination, or other contamination.
DNA plasmid, spike protein, and bacteria are all known to activate cGAs STING pathway.
This pathway can be hyperactivated
4/ of in one or more areas it is bombarded repeatedly with virus (spike), dsDNA (double stranded DNA, like DNA Plasmid), bacteria, and can also become dysregulated if STING 3 variant is there, a mutation, other TMEM173 variant, etc. nature.com/articles/s4143…
TMEM173 variants and potential importance to human biology and disease - Genes & Immunityhttps://www.nature.com/articles/s41435-018-0029-9
5/ Scenario: young teen, gets covid vaccine. spike protein (and or DNA plasmid or other contaminant) bombards cGAs STING pathway (STING variant may be present/and or mutation in STING):
Mechanism of cGAS-STING Activation and Hyperactivation
1. cGAS-STING Pathway:
6/ cGAS: Detects plasmid DNA/spike and produces cyclic GMP-AMP (cGAMP).
STING (Stimulator of Interferon Genes): Activated by cGAMP, leading to the production of type I interferons and other pro-inflammatory cytokines.
7/ This will lead to chronic activation and positive forward/Feedback loop and hyperactivation of this pathway.
Continuous presence of DNA plasmid pieces in the cytosol due to their repeated introduction or inefficient clearance/with spike protein.
8/ this results in sustained activation of the cGAS-STING pathway, resulting in a chronic inflammatory state.
This generates more cells that are nearby to become involved due to paracrine diffusion.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10960729/
9/ Persistent inflammation causes continuous tissue damage, releasing more cellular debris and DNA, further activating the cGAS-STING pathway. The cells that are getting attacked by the body's own immune system release their OWN DNA , and this activates cGAS again, and the
10/ body comes back in and attacks a second time, and begins a LOOP of attack, a cycle, and it continues (many other threads on this starting back in October).
11/ then the neuroinflammation begins of the brain and central nervous system.
High levels of inflammatory cytokines can cross the blood-brain barrier (BBB), leading to inflammation in the central nervous system (CNS).
12/ Chronic inflammation can activate microglia (the brain's resident immune cells), causing them to release neurotoxic substances.
This is all a cascade occurring from the cGAS STING pathway being activated, and becoming dysregulated as it is activated.
13/ cGAS is supposed to protect us.
Demyelination and neuronal damage now starts to occur. Nerves are now being damaged, and neurons in the brain are being attacked by the body's immune system.
14/ Chronic inflammation might trigger an autoimmune response against neural tissues, leading to demyelination (loss of the protective myelin sheath around nerves) and neuronal damage.
15/ this prolonged inflammation and immune attack can result in axonal injury, disrupting neural communication--the communication with the brain to other parts of the body--like the arms and legs.
Inflammatory myelopathy might begin.
16/ : Inflammation of the spinal cord (myelitis) can cause damage to the neural pathways responsible for motor control--this is muscle control.
Them in the case of paralysis. severe inflammation can lead to swelling or compression of the spinal cord,
17/ impairing neural transmission.
Inflammatory damage to peripheral nerves can result in neuropathy, affecting muscle function and leading to weakness or paralysis.
I am so sorry.
18/ If occurring over a couple of weeks:
Early Symptoms:
Weakness including Initial weakness in limbs, difficulty walking, or muscle fatigue.
Numbness, tingling, or pain in affected areas.
19/ Progressive loss of motor control, leading to difficulty with movement and coordination.
Complete loss of voluntary muscle function in affected regions, potentially leading to paraplegia or quadriplegia depending on the extent of spinal cord or nerve involvement.
20/ reason for teens to suffer from stronger damage is stronger immune response due to age. Adolescents may have a more robust immune response, which could result in an exaggerated reaction to the presence of DNA plasmid and spike protein with contaminants and/or sting variant
21/ younger nervous system. Puberty involves significant hormonal changes, which could modulate immune responses and contribute to a more intense inflammatory reaction.
22/ What happened:
The persistent presence of DNA plasmids and or spike with STING variation maintains the activation of the cGAS-STING pathway, causing a cytokine storm with high levels of inflammatory mediators in the bloodstream.
23/ Pro-inflammatory cytokines cross the blood-brain barrier, leading to inflammation within the central nervous system (CNS). Microglia (resident immune cells in the brain and spinal cord) become activated and start releasing neurotoxic substances.
24/ Inflammation spreads to the spinal cord (myelitis), causing swelling, edema, and damage to neural tissues, attack the myelin sheath, leading to demyelination, disrupting neural signal transmission.
And then, axonal damage and permanent damage to the central nervous system
25/ paralysis
************************************************************************************
There's been much talk about P53 (tumor suppressor gene) and the role of the spike protein and DNA plasmids in the action of this gene. Well, p53 doesn't just waive a magic wand. p53 acts on the cGAS STING pathway in tumor suppression. cGAS STING has multiple roles in both driving cancer and stopping it.
*********************************************
********************************************************************************************
cGAS STING pathway is the new target, and not just cancer. There's a reason why hydroxychloroquine works with lupus and other autoimmune conditions (hydroxychloroquine works on the cGAS STING pathway). Same goes with cancer, ulcerative colitis, stroke, and myocarditis.
************************************************************************************
***********************************************************************
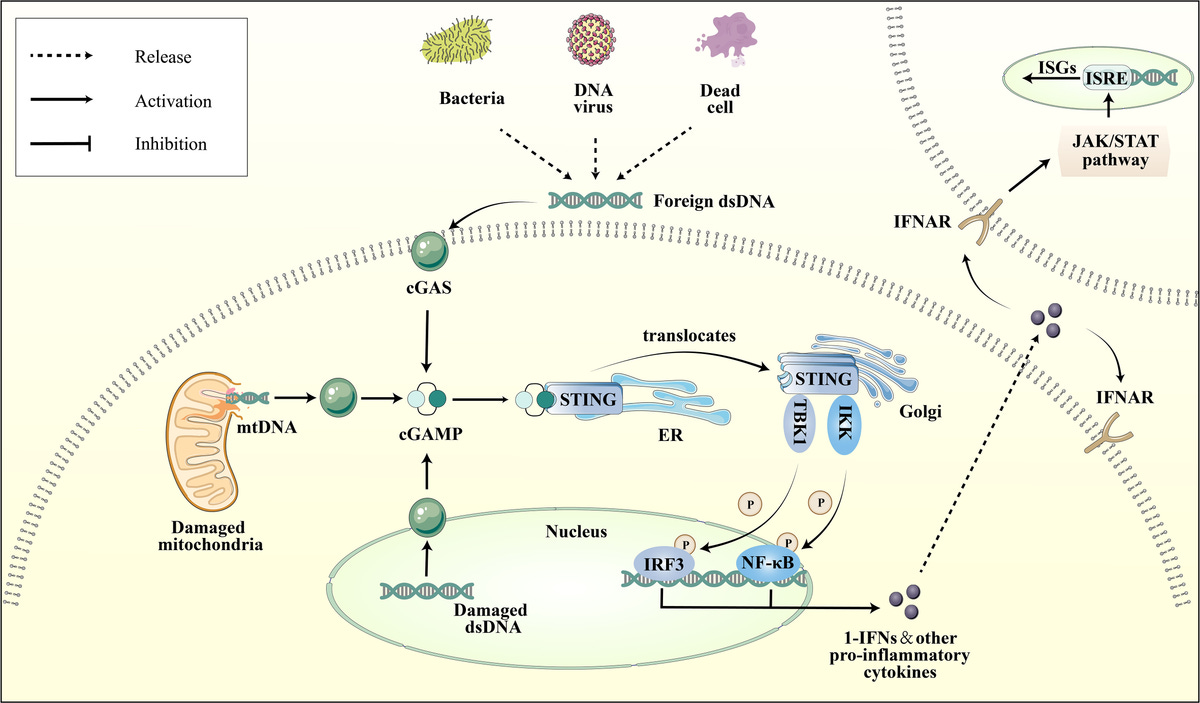
How plasmid DNA will track to the nucleus (with extra steps) before it gets degraded in the cell. First it gets recognized by cGAS, but that is not the only thing that will recognize it, and actually PROTECT it from being broken down, and zip it right to the nucleus.
DNA plasmid will be recognized by the cGAS STING pathway immediately upon cell entry. This is the first part of the cell that recognizes it with rapid speed. cGAS is the cell's main "smoke detector" that recognizes things that do not belong, like viruses, bacteria, double stranded RNA and DNA.
But here's the thing--DNA will cause the strongest response, and it is also LENGTH dependent--longer pieces of DNA plasmid will cause cGAS STING to cause the strongest response of all, even at lower amounts.
You can have a mixture of different lengths of DNA. Small pieces will activate the immune system in a mold to moderate way. Medium sized pieces of DNA will trigger a stronger response--the small pieces will actually act as "pass interference" (US football term here) and stop the larger pieces from binding.
The longer pieces of DNA will cause a very strong immune system response--one that can be dangerous, and cause a hyperactivation of our immune systems and cause it to attack us, destroy tissue, and cause us injury, including but not limited to autoimmune concerns.
There is also an order to the organs in our body (eyes, brain, bladder, heart, etc) with how other things will impact the cellular damage (I am explaining on podcasts coming up, and why myocarditis (heart damage) aside from immune issues and clots, are the highest injuries seen (followed by bladder, etc).
Back to what is happening in the cell. There is an order of operations in our cells for how things are taken care of regarding foreign entry of plasmid DNA. Right now we will just speak to entering the nucleus.
There are multiple proteins inside the cell that can bind to plasmid DNA, and some do not care what kind of DNA, they see it as "bad" because DNA does not belong free floating around in our cells.
The proteins:
✅cGAS (cyclic GMP-AMP synthase)
✅IFI16 (Interferon Gamma Inducible Protein 16)
✅Other DNA-Binding Proteins (like HMGB1 (High Mobility Group Box 1) and DDB2 (Damage-Specific DNA Binding Protein 2)
✅Motor Proteins (DYNEIN!!!!!)
And then we have the thing that breaks down the DNA plasmid:
✅TREX1
☑️1. DNA plasmid has now entered the cell vis a lipid nanoparticle
☑️2. Initial Interactions with cGAS
cGAS-STING Pathway (IFI16 as well):
cGAS STING and IF116 rapidly detect cytoplasmic DNA, particularly double stranded DNA (like plasmid DNA).
IFI16 specifically senses foreign DNA and activates immune responses similar to cGAS-STING.
The cGAS-STING pathway is known for its rapid response to foreign DNA, triggering signaling cascades within minutes of detection. cGAS STING, the smoke detector for our cells, is coming in fast and hard, and is the first on the scene.
cGAS (the smoke detector) has the evolutionary advantage in binding to newly arrived cytoplasmic DNA to trigger an immune response quickly.
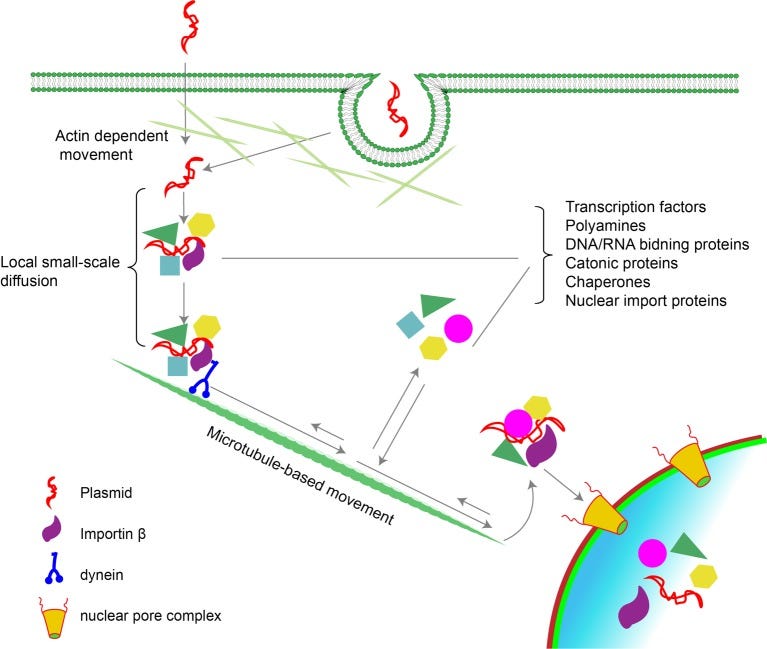
☑️3. Dynein time!
Dynein is going to come in with rapid speed too--about the same time cGAS does, or just behind it. Dynein is what is called a MOTOR PROTEIN!
It looks like this little ball with feet, and it can attach to plasmid DNA, it will bind to it! It will attach to the DNA plasmid, and carry it on its back like Luke Skywalker carrying Yoda (except on a tight rope to the nucleus, it is now walking it to the nucleus).
(see video below of Kinesin protein walking on a little rope thing we call microtubules, it will look like it is walking on a tight rope. It is simlar to Dynein).
However, when Dynein binds to plasmid DNA it is now protected from being broken down! It is now shielded, like Luke Skywalker shielding Yoda from harm.
☑️4. Size of DNA plasmid matters!
Size Variation and Transport Efficiency:
Small Plasmids (20-120 base pairs):
Smaller plasmid fragments diffuse more readily and are easily transported by dynein-microtubule interactions. T
Their smaller size allow them to move through the nuclear pore complex more easily.
Medium Plasmids (120-2000 base pairs):
Medium-sized plasmids benefit significantly from active transport mechanisms.
Their interaction with dynein and microtubules ensures they reach the nucleus without being degraded or immobilized within the cytoplasm.
Large Plasmids (2000-4000 base pairs):
Larger plasmids might face more difficulty due to their size but are still effectively transported by the cytoskeletal network.
☑️5. Dynein can bind to plasmid DNA either directly or via adaptor proteins that recognize the DNA.
Dynein moves the DNA towards the cell center (minus end of microtubules), directing it towards the nucleus.
☑️ NO SV40 needed to get it into the nucleus, there are CHARGE MEDIATED localization reactions here--meaning the CHARGE is driving it into the nucleus!
The negative charge of DNA, due to its phosphate backbone, can interact with positively charged molecules or proteins within the cell.
☑️6. Another way DNA plasmid can enter the nucleus During Telophase
Our cells are dividing, and when this happens, DNA plasmids can enter the nucleus.
Cell Division Phases
Prophase: Chromosomes condense, and the nuclear envelope begins to break down.
Metaphase: Chromosomes align at the cell's equatorial plane.
Anaphase: Chromatids separate and move to opposite poles of the cell.
Telophase: Nuclear envelopes re-form around each set of chromosomes, and the cell begins to split into two daughter cells.
The nuclear envelope is more permeable during reformation, allowing easier entry of exogenous DNA.
The temporary absence or incomplete formation of the nuclear envelope reduces physical barriers to DNA entry.
☑️ 7. So now, we have cGAS that has bound to DNA plasmid, and we have DYNEIN that has walked the DNA plasmid pieces right to the nucleus, carrying it like a snuggie on its back.
☑️ 8. TREX 1 comes into the picture. TREX 1 is the thing that is going to break down DNA plasmid, but Dynein-bound DNA is likely less accessible to nucleases because it is associated with a large protein complex. TREX has a difficult time interacting with the motor protein carrying the DNA plasmid on its back.
☑️9. Association with other proteins!
In addition to dynein, plasmid DNA can associate with other cellular proteins that shields it from degradation. These proteins can form complexes that obscure the DNA from nucleases like TREX 1.
HMGB1 (High Mobility Group Box 1)
Can bind DNA and influence its stability and interaction with other cellular components.
Acts as a DNA chaperone and can also participate in the immune response.
IFI16 (Interferon Gamma Inducible Protein 16)
A DNA sensor that can detect foreign DNA in the cytoplasm.
Activates immune responses, similar to the cGAS-STING pathway.
DDB2 (Damage-Specific DNA Binding Protein 2)
Involved in DNA repair and can bind damaged DNA.
Participates in recognizing and responding to DNA damage.
☑️10. Limitations of cGAS-STING Pathway
The cGAS-STING pathway can become saturated if there is an overwhelming amount of dsDNA in the cytoplasm. Saturation occurs when the amount of DNA exceeds the pathway's capacity to process and respond effectively.
The response of the cGAS-STING pathway is dose-dependent, meaning that higher concentrations or continuous exposure to dsDNA can lead to prolonged or sustained immune activation. This can potentially lead to chronic inflammation or immune dysregulation, and damage to organs causing injury to people, and autoimmune disease.
The ability of cells to handle dsDNA can vary between different cell types and under different physiological conditions. Some cells may have higher tolerance thresholds for dsDNA, while others may be more sensitive (I will explain this on the podcast coming up!)
☑️11. cGAS can have altered functionality for a few reasons. This has been discussed in other threads, including by RACE (your RACE determines what kind of cGAS you have, er smoke detector in your body! There are also what are called HLA mutations (DR) that can cause a dysregulation in immune response.
Genetic Mutations: Mutations in genes encoding cGAS, STING, or associated proteins can impair the pathway's ability to detect or respond to dsDNA properly.
Certain diseases or conditions may interfere with cGAS-STING signaling, either enhancing or dampening its response to dsDNA. For example, viruses may encode proteins that inhibit or exploit cGAS-STING signaling to evade immune detection.
Drugs or therapies that modulate immune responses, including those targeting cGAS-STING pathway components, can also alter immune responses in desired or undesired ways.
☑️12. So lastly, TREX 1 comes in and this is the thing that cleans up DNA, and it is the last thing to arrive on the scene in your cell, after cGAS has already had its way with it, after the immune system has already been activated, and after the plasmid DNA has already been transported to the nucleus. It is the slowest moving part of your cell's defense mechanism.
Here is what a motor protein looks like walking along a microtubule, this is Kinesin, not Dynein, but it will carry that DNA plasmid like Luke carrying Yoda on its back, and it will walk it right to the nucleus
The DNA can also get in via diffusion, it can just transfer right into the nucleus via charge mediated transport, meaning the DNA has an ATTRACTION to things in the nucleus it wants to join with, it wants to be with.
The Dynein protein, will carry the DNA plasmid like yoda in its back and walk it right to the nucleus, and it will be protected from TREX1 , the thing that is trying to break down the DNA in the cell.
*************************************************************************
**************************************************************************
DNA plasmid pieces hit cGAS to cause inflammation and genomic instability, but in the pancreas we have what's called DUOX2. Exogenous DNA, like DNA plasmid contamination, can interact with DUOX2, which can drive multiple cancers forward like pancreatic cancer, colon cancer, thyroid cancer, and others, which activates the cGAS STING pathway, AND the AKT pathway, while causing the upregulation of APOBEC enzymes, which can also lead to genomic instability, potentially causing further mutations in critical genes outlined in the multi hit theory of cancer by Vogelstein, promoting the expression of cancer, even when a starting point was, pancreatitis. There is also another parallel mechanism at play here when certain injections are involved which increase inflammation, causing cells to release their own DNA, like mitochondrial DNA, the NETs, and nuclear DNA, hitting cGAS STING AGAIN. This is all without insertional mutagenesis of DNA plasmid integration which is a whole different nightmare.
***********************************************************************