Lipid Nanoparticles of Specific Size and Charge Can Enter the NUCLEUS Without DNA/NLS or SV40, Interact with Histones, and Cause DNA Mutations--A STUDY--NOT A HYPOTHESIS
CHARGE and SIZE of the LNP ARE the Nuclear Localization Signals
The first time I wrote about charges in a Substack (I was blasting this stuff out on Twitter prior).
First Podcast on LNP, recombinant proteins, toxicity, and how the physical time and processes done in Warp Speed to bring these things to market, just cannot be accurate:
We hit the charges hard on this one:
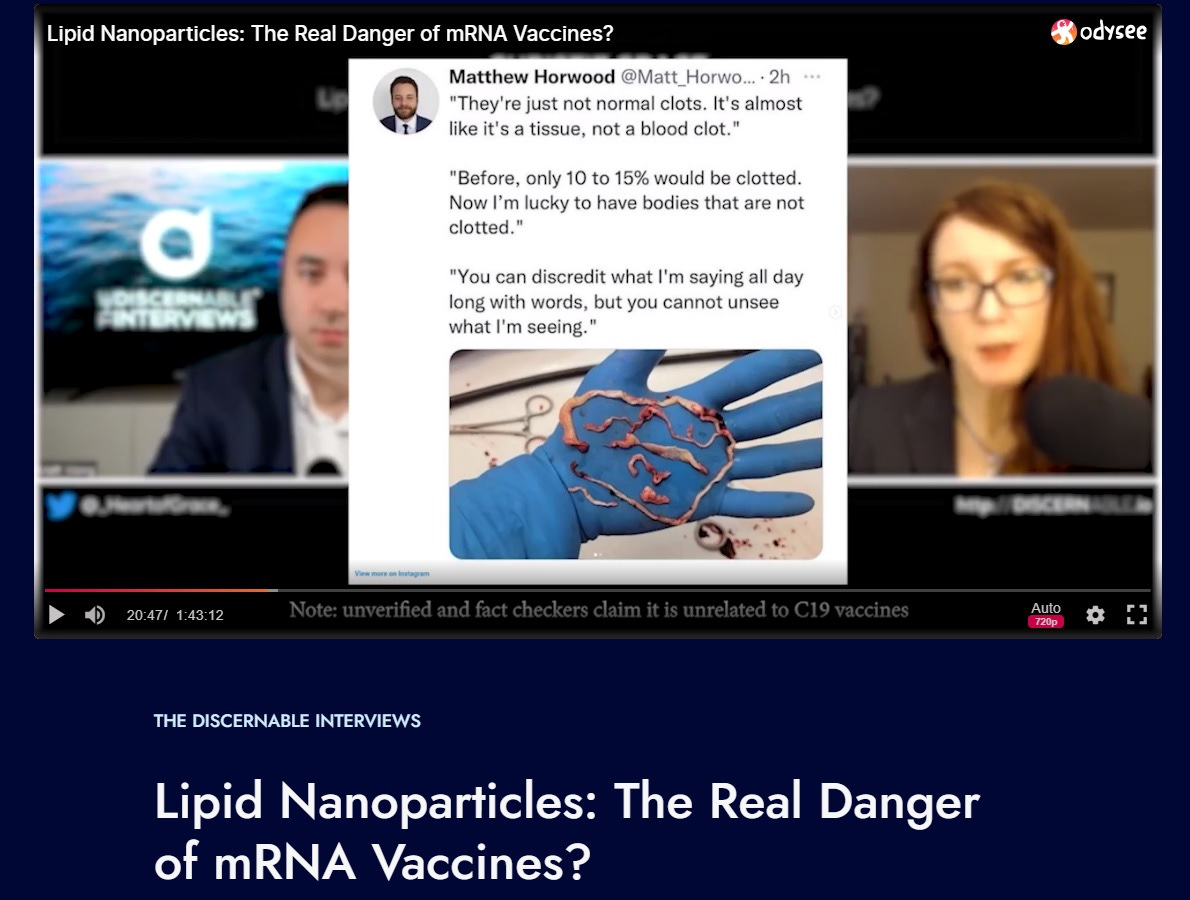
Lipid Nanoparticles: The Real Danger of mRNA Vaccines?
I was speaking out a year ago on the mechanism driving these thick clots. Thanks to those of you who have stuck by my side. That means a lot. Thank you.
Thank you Dr. Pierre Kory, Dr. Drew Pinsky, the podcasters who had me on, and others on Twitter, for sharing my threads, and supporting me.
Thank you to those in Germany for all you are doing, and France. So many do not hear about you and your work. Thank you.
Back in August of 2023 on a conference hosted by Vejon Health, I stated the elements of the LNP were able to get into the nucleus, and due to charges, damage and mutations could occur. I also spoke about clots, charges in the lipids forming adducts and frameshifting, etc.
Vejon Health: Post Vax/Long COVID Congress – The Silent Disaster
************************************************************************************************************
Now we are going to look at a study, that proves this. I will assist and add descriptors, and more data, with citations.
The study (which will translate right over to LNP, quite perfectly) will show you how, because a charge is a charge):
DOI: 10.1002/smll.200800088
CdTe Nanoparticles Display Tropism to Core Histones and Histone-Rich Cell Organelles
Jennifer Conroy,* Stephen J. Byrne, Yurii K. Gun’ko, Yury P. Rakovich, John
F. Donegan, Anthony Davies, Dermot Kelleher, and Yuri Volkov
This is a difficult study to find, so I am giving you the Sci Hub link.
There is this link too: https://onlinelibrary.wiley.com/doi/10.1002/smll.200800088
The researchers used what are called Quantum Dots, and they used cow’s blood. The same will hold true in human blood.
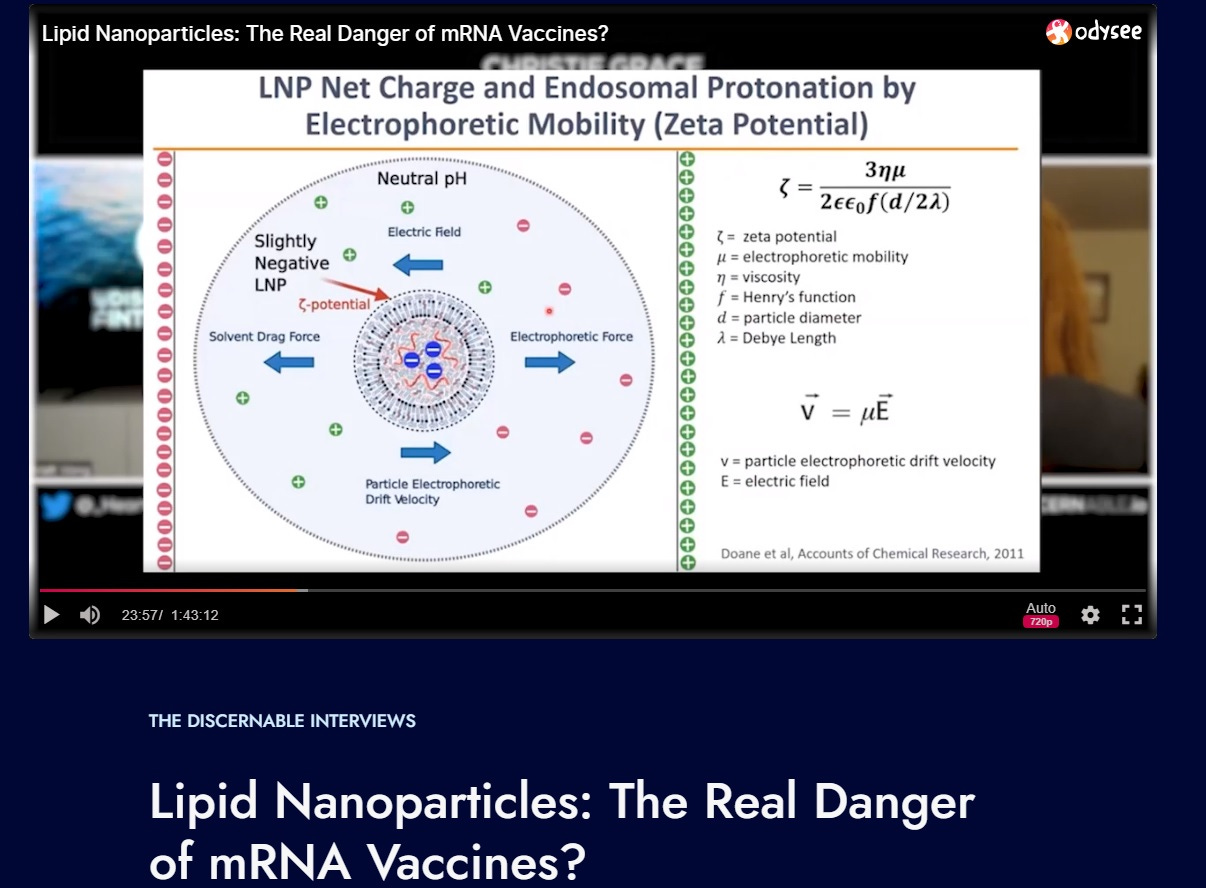
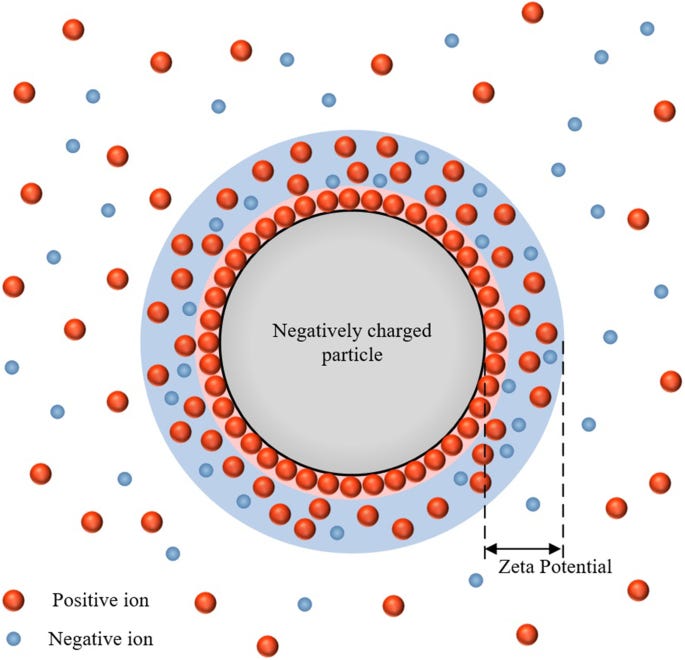
The Quantum Dots have a size, and a zeta potential.
(Look back at tweet threads or other Substacks for zeta potential learning):
Here is every thread I can think of for those who do not want to be on X (Twitter), or want them all in one spot:
https://threadreaderapp.com/user/_HeartofGrace_
Here are the basic elements:
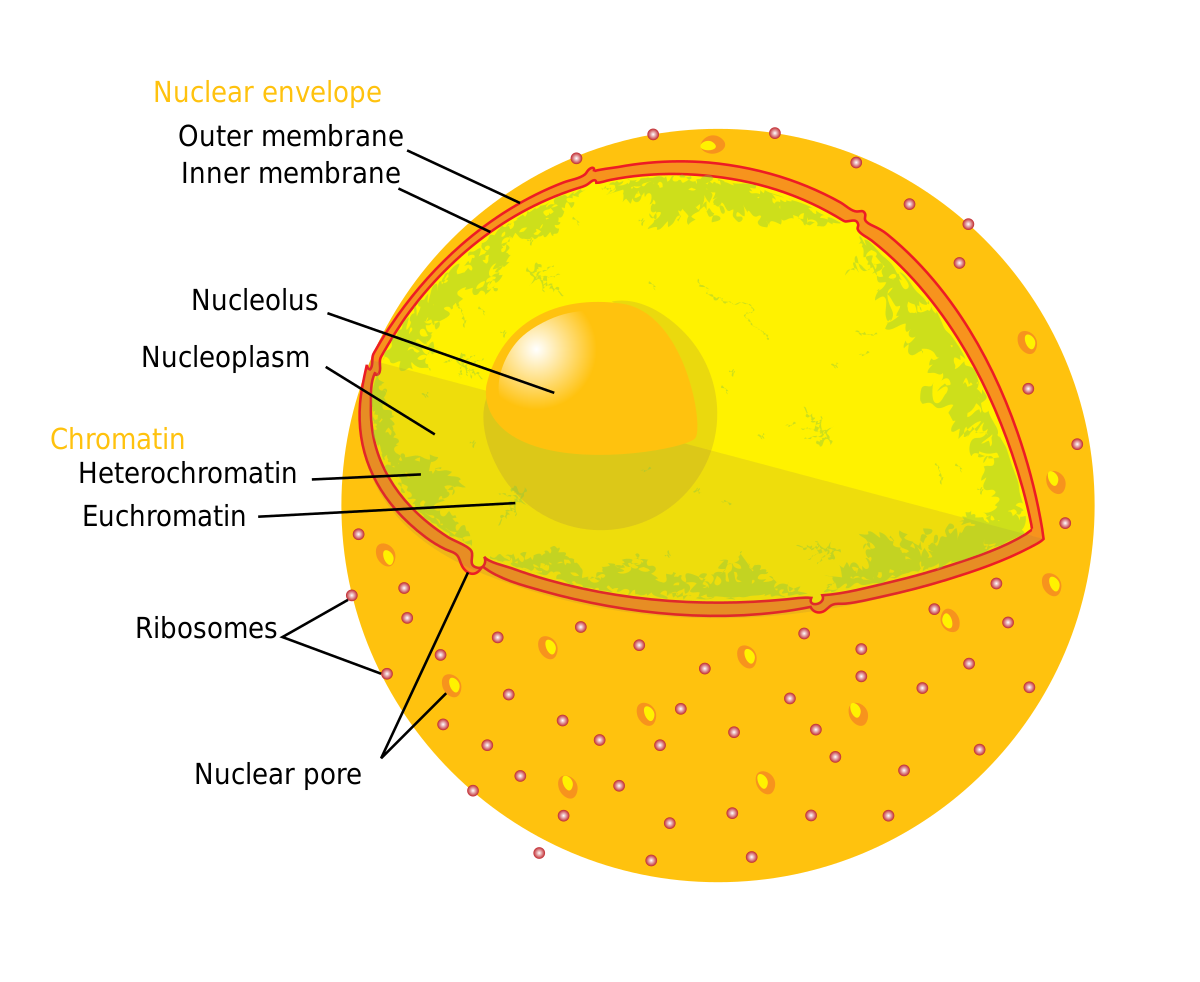
Nuclear pore information regarding the nucleus of a cell
Although some quite wide variations in pore sizes have been reported, the diameter of the pore proper is usually about 55–70 nm while that of the outer rim of the annulus is about 100–120 nm.
This means molecules or particles smaller than the diameter of the “pore proper”, around 55 to 70 nm, have a higher likelihood of freely diffusing through the nuclear pore complex and entering the nucleus. This means they will just flow right in. This is what diffusion means—they will pass right in.
However, larger particles may still pass through under certain conditions, and the actual size limit can vary based on factors such as the specific properties of the particle, the cellular state, and interactions with transport proteins—and CHARGE.
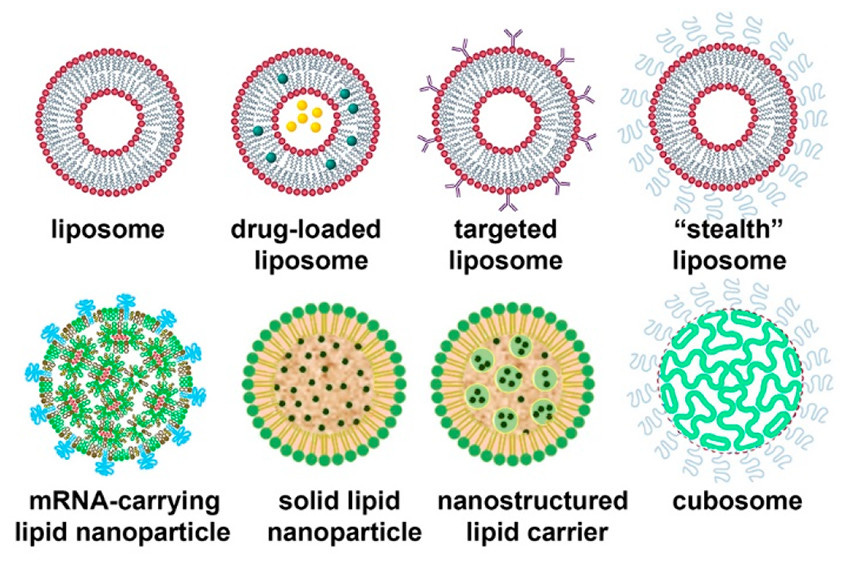
To blend this study and other information, the lipid nanoparticles in the current “modRNA vaccines” vary quite a bit in size. They do not have much uniformity in them, not in PEGylation, charge, other composition such as molar ratio, amounts of DNA plasmid contamination versus RNA, etc.
Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement
“for the Pfizer and (50:1.5:38.5:10) for the Moderna vaccine. Those nanoparticles are 80–100 nm in diameter and contain approximately 100 mRNA molecules per lipid nanoparticle.)”
Other studies show the LNP is more variable in size, from 50 nm to 200 nm.
That means there are some LNPs in this size range that will, according to size, be able to diffuse right into the pore. But that is not all that is needed.
Here are the other elements of the study:
Quantum dots were used to see how they interacted with cells. They went RIGHT into the nucleus! They do not have DNA in them, or RNA! They have NO lipids about them at all. How did they do it without SV40, or DNA plasmid? how did they do that?
Quantum Dots= QD.
CHARGE and SIZE!
Basic information:
Starting Zeta Potential of QDs:
The initial zeta potential of the QDs was -28.9 mV.
Ending Zeta Potential of QDs (the charge changed!):
After interaction with core histones, the charge shifted from negative to positive, reaching +15 mV.
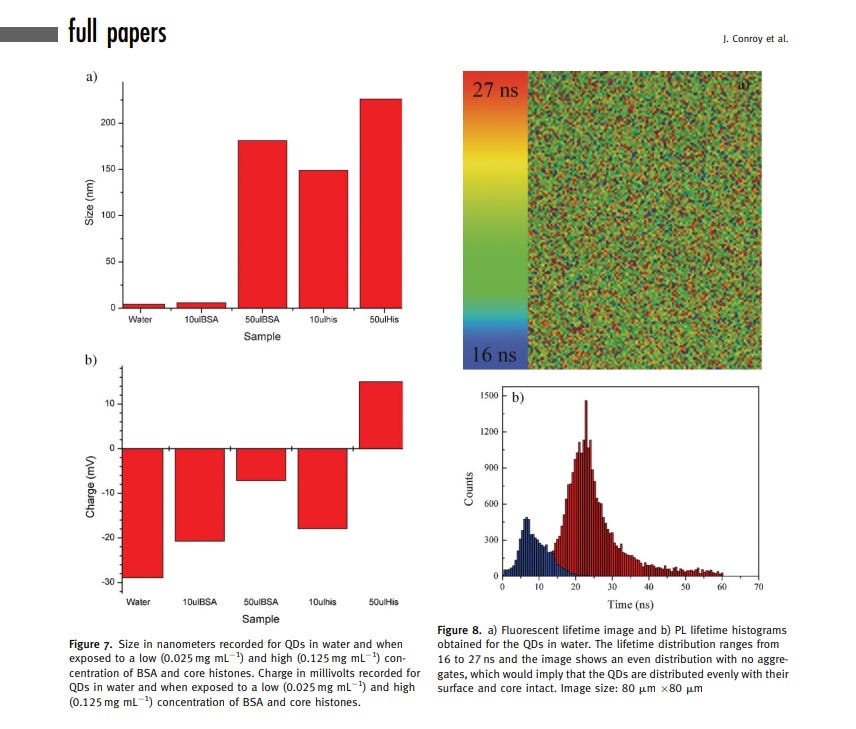
Starting Size of QDs (what?):
The initial hydrodynamic diameter of the QDs was 3.4 nm.
Ending Size of QDs:
After interaction with core histones, the size increased to 225 nm.
Entry into the Nucleus:
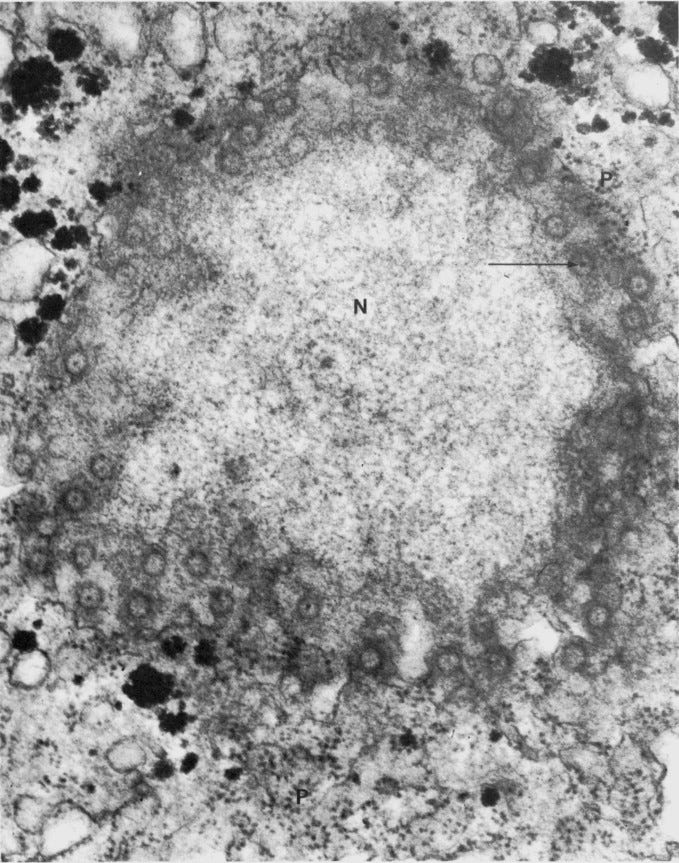
Confocal microscopy demonstrated that the negatively charged QDs entered the nucleus and nucleoli of THP1 cells.
Behavior in the Nucleus:
Upon entering the nucleus, the QDs exhibited tropism towards core histones, with preferential binding observed.
Interaction with Histones:
The QDs showed strong binding affinity to core histones, which are positively charged due to the presence of lysine and arginine amino acids on their N-termini.
Effect on Histones:
The interaction with QDs induced significant changes in histone behavior, including aggregation and potential alterations in their biochemical properties.
First, we will break down this study further, show images, speak to mechanisms, and then we will flip it over to LNP. You do NOT get to say “we need to show this with LNP”. NO. CHARGE IS A CHARGE! ANYONE making this excuse, is making an excuse at this point!
These are quantum dots, and they are small, but they get bigger once they enter the nucleus. They have quite a small size, but they only need to be in the size range of the nuclear pore to get through, because that is not, other than size, how they are getting pulled in, mostly.
The quantum dots, are getting pulled right into the nucleus, and not interacting with other areas in the cells, because they have a negative charge of almost - 30 millivolt (if anyone reads my twitter posts/threads right now, you know that -30 millivolt is a HOT number that comes up a lot, because it is the charge that may cause clots, that may cause those thick spindly clots a little lower will do it too), it may cause the LNP to transfect the cardiomyocyte, and it may cause in smaller size and that charge, to transfect lymphocyte).
We need to talk about histones for a moment.
Histones have a high positive charge. The QDs have a high negative charge. That means, if the particle with a very negative charge manages to get into a cell, it will be VERY attracted to the positively charged histones, and in this study, that is exactly what they did.
Histones:
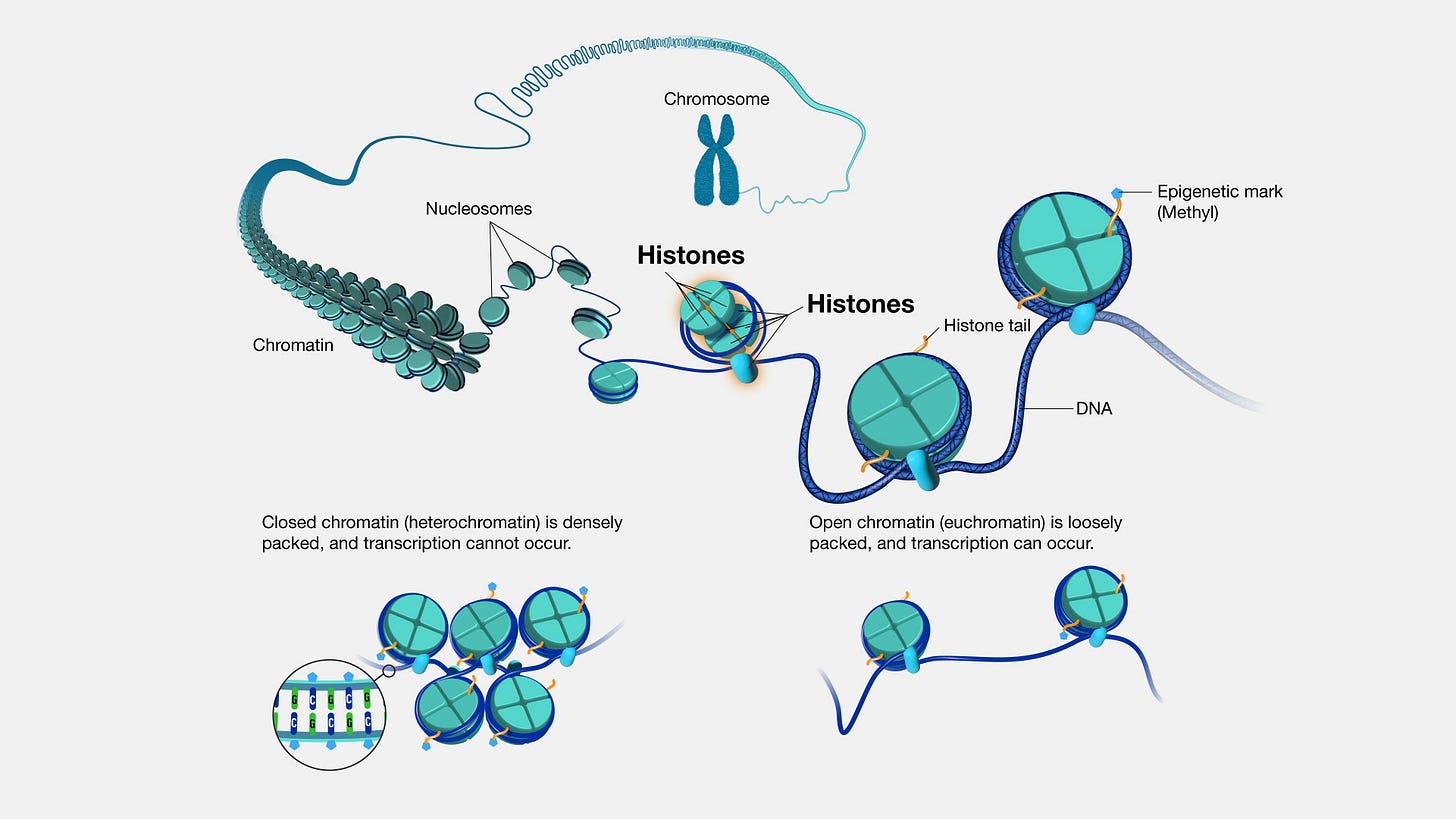
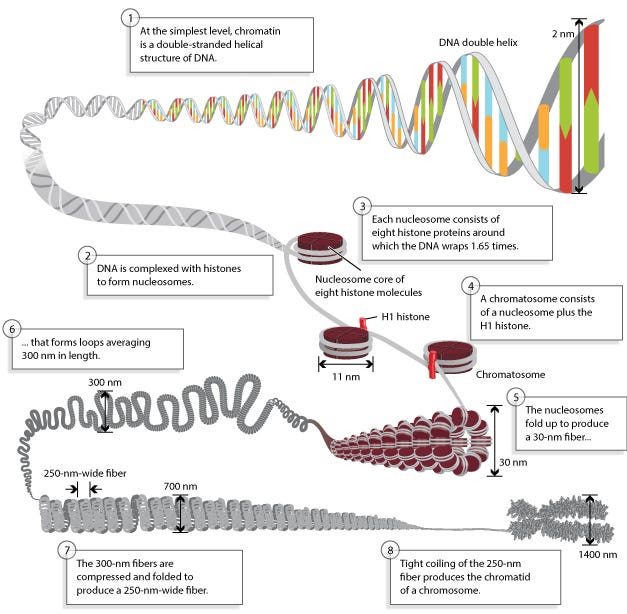
Histones are a class of proteins found in eukaryotic cells, our human cells (not bacteria), associated with DNA in the cell nucleus. They are highly positively charged due to their composition of basic amino acids—lysine and arginine residues.
Histones package and organize DNA into a compact structure called chromatin, which condenses to form chromosomes during cell division.
If you dismiss this, I do not know what to tell you.
There are five main types of histones: H1, H2A, H2B, H3, and H4, which form the core histones around which DNA is wrapped to form nucleosomes.
Nucleosomes consist of DNA wrapped around a core of eight histone proteins, composed of two copies each of histones H2A, H2B, H3, and H4.
The positively charged amino acids of histones interact with the negatively charged phosphate groups of DNA, facilitating its packaging and compaction.
(if this is getting way into the science weeds, at the end of the day, we are going to focus on size, charge, and what the bad things are)
Histones are primarily located in the cell nucleus, where they interact with DNA to regulate gene expression, chromatin structure, and various cellular processes.
By compacting DNA into chromatin, histones help regulate access to the genetic information encoded in DNA.
They can also undergo various post-translational modifications, such as acetylation, methylation, phosphorylation, and ubiquitination, which can alter chromatin structure and influence gene expression.
When quantum dots (QDs) interact with histones, several implications arise.
The highly positively charged nature of histones may attract negatively charged QDs, leading to their association and altering the physical and chemical properties, of the HISTONES.
This could affect the behavior and function of both histones and QDs, influencing chromatin structure, gene expression, and cellular processes regulated by histones.
Histones are primarily localized in the nucleus and associated with DNA.
QDs AND LNPs that have a high negative charge, interacting with histones have a higher propensity to enter the nucleus and interact with chromatin.
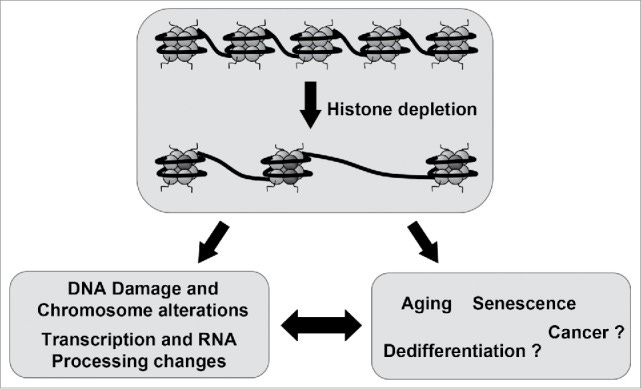
This could affect nuclear processes, such as DNA replication, transcription, and repair, potentially leading to cellular dysfunction or perturbation of normal physiological processes, and lead to , toxicity, disease, aging, and CANCER.
Back to the study (we are going to jump in and out of it to explore more in-depth mechanisms):
The mechanism of nuclear localization: : a strong tropism of quantum dots (QDs) to nuclei and nucleoli . Tropism means an affinity for that spot, it is going there, selectively.
This, the nuclear localization, is mediated by charge-related properties of macromolecules present in these nuclear compartments—namely, the positively charged histones drawing in negatively charged QDs. The same should occur, on the charge and size basis alone, to an LNP that fits this size, and zeta potential (charge).
Using a human phagocytic cell line (THP1), nuclear lysates, purified proteins, and nucleic acid solutions, the study investigates the interactions of QDs with intranuclear macromolecules—namely parts of the cell that include histones, and other portions of it.
The findings indicate that unmodified QDs preferentially bind to positively charged core histone proteins, although they still are able to interact with DNA or RNA.
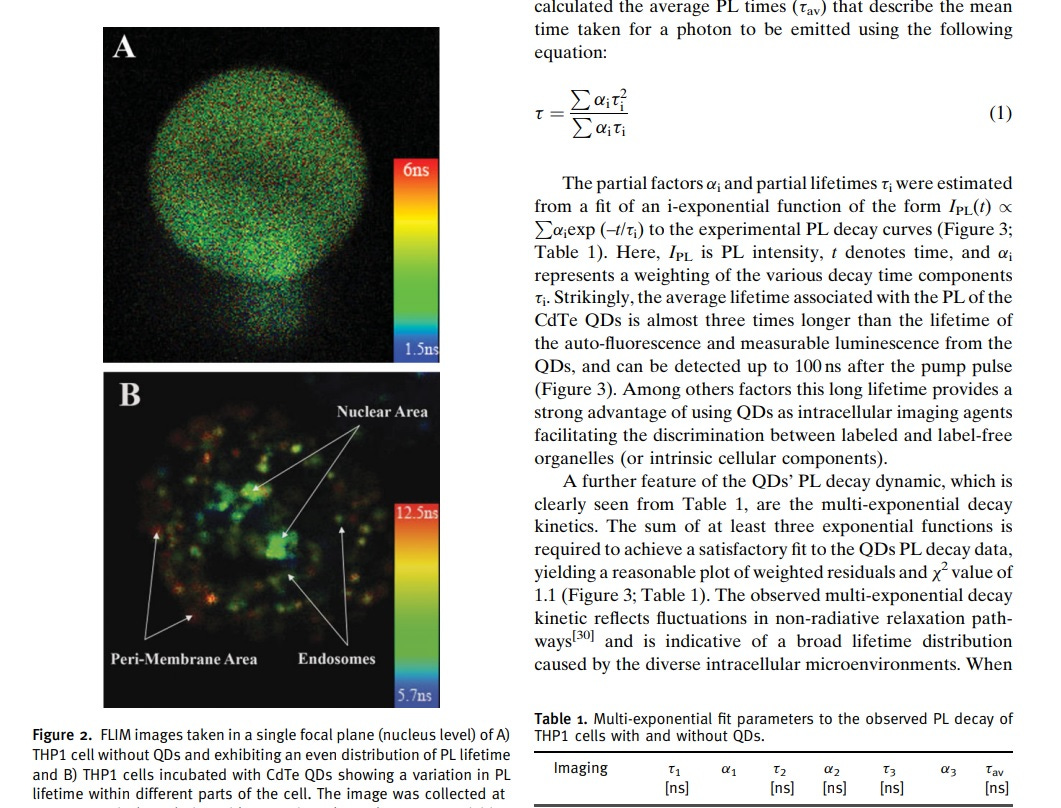
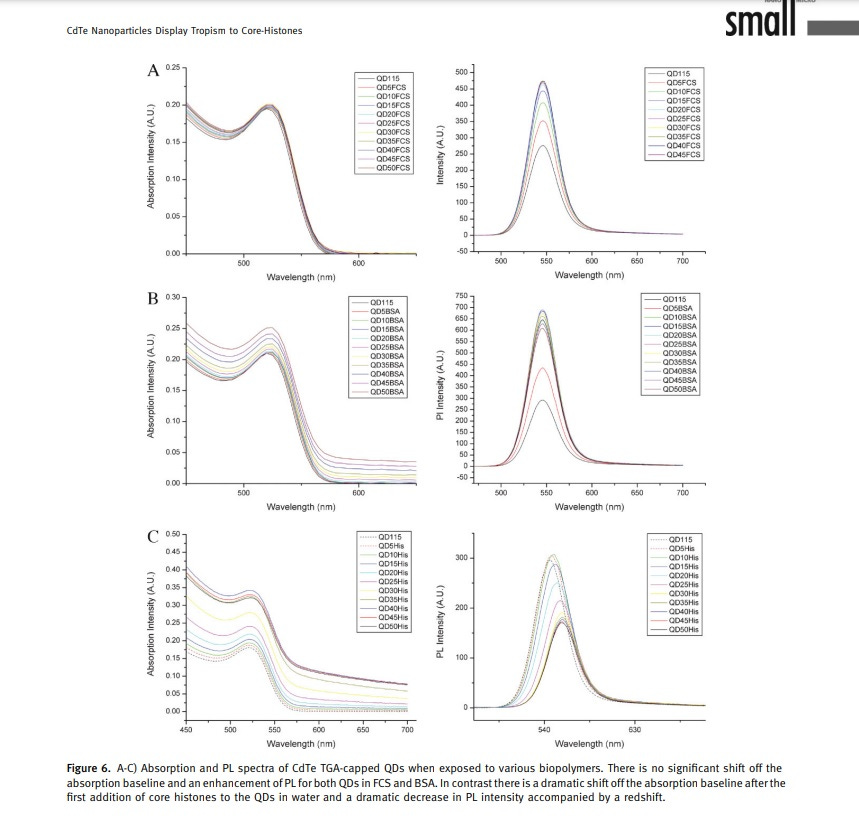
From an imaging standpoint, the interaction with core histones leads to a dramatic shift in the absorption band, a redshift, and a decrease in the photoluminescence (PL) intensity of the QDs.
Fluorescent lifetime imaging (FLIM) demonstrates an increased formation of QD/protein aggregates in the presence of core histones.
!!!!
Small green-emitting negatively charged (QDs) demonstrate rapid accumulation in the nucleus of phagocytic cells.
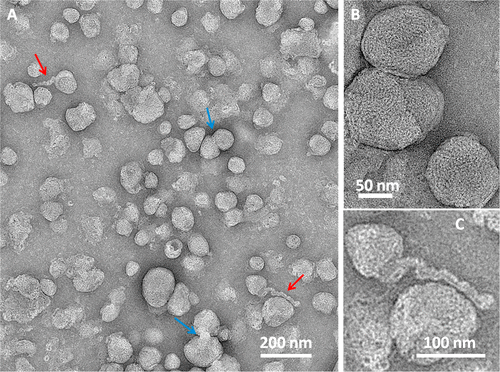
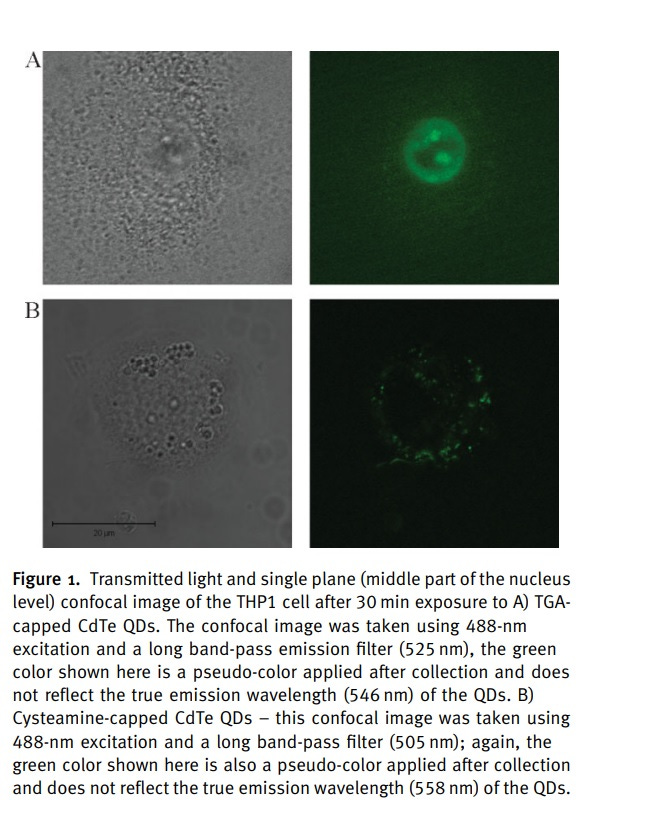
Figure 1A illustrates the accumulation of 3.2-nm-sized thioglycolic acid (TGA)-capped QDs in the nucleus and nucleoli of THP1 cells after 30 minutes of incubation.
The fluorescence pattern indicates predominant membrane-associated fluorescence and discrete cytoplasmic aggregates of cysteamine-capped CdTe QDs (positively charged) following the same incubation period, as shown in Figure 1B.
However, no nuclear or nucleoli accumulation is observed with the positively charged QDs.
Experiments with cysteamine-capped QDs rule out particle size as the reason for the differing ultimate locations within the THP1 cells. This means surface charge of the QDs determine the cellular uptake mechanism and their ultimate location within the cell.
The size was not making things change where it went—it was the negative charge, as long as it was within the size to where it could get through a nuclear pore.
There was no DNA, NO SV40, NO separate NLS (nuclear localization signal) mechanism.
It went in due to charge.
Aggregation then occurred, which did and was shown in this study.
The QDs aggregate within the nuclear and nucleolar compartments due to their interaction with biopolymers present in abundance. Biopolymers are proteins and nucleic acids, like DNA. Human DNA. Stem cell DNA. Germline DNA.
What the study also found, is the change in the zeta potential, meaning the charge of the QD went from NEGATIVE to POSITIVE.
Charges do not just disappear.
For the zeta to change from -28.9 millivolt on the quantum dot to positive 15 millivolt, means the ELECTRONS went somewhere.
ELECTRON TRANSFER OCCURED.
When the negatively charged particles enter the nucleus, and when they are in close proximity to intranuclear organelles, such as histones or other proteins, electron transfer reactions may occur between the photoexcited semiconductor nanoparticle and cations bound at the surface of these organelles. This can change the complete processes, activities, and structure of these things, including histones.
The electrons from the excited QDs would transfer to the cations on the surface of these biomolecules, resulting in a chemical reduction of the cationic groups present on the surface of the organelles, like the Histones.
You better believe it. And this can happen with the LNP as well.
If a lipid nanoparticle with a negative charge of even at least -10 millivolts and a size of 60 nm were introduced into the cellular environment, it would likely interact with intracellular components in a manner similar to the QDs.
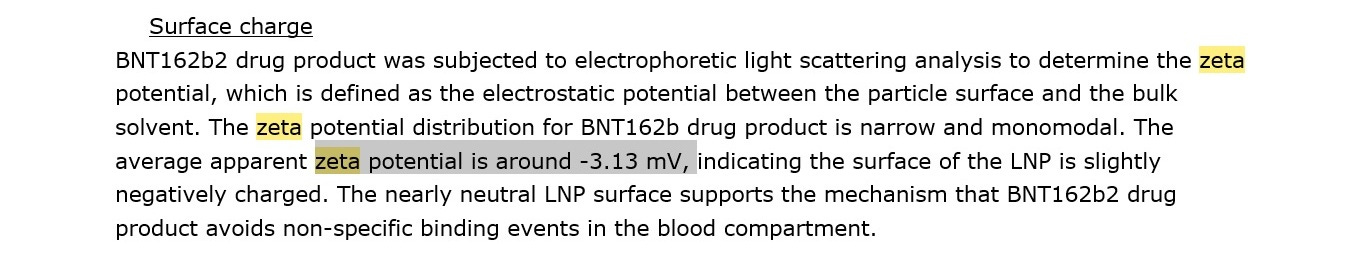
Pfizer stated the starting zeta potential in their own documents (thanks Mckernan) was approximately -3 millivolt when it was tested using process one, which did not contain any DNA plasmid. The thing is, zeta potential changes in pH, when viscosity changes, ions present in blood, etc, and even without DNA plasmids present as contamination, the zeta potential would shift right away when entering the human body to about -8 millivolt.
^^^Pfizer’s own document.
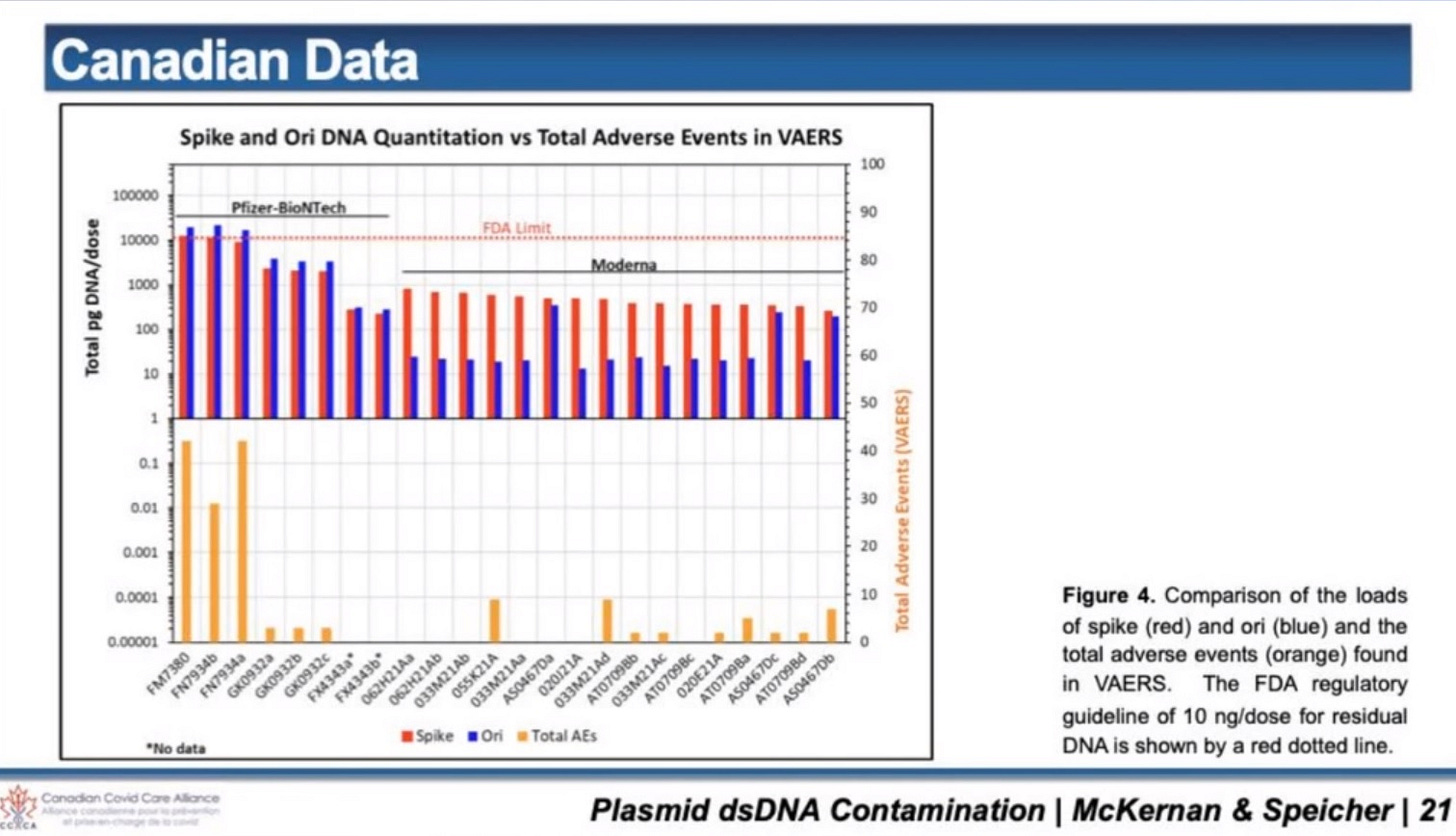
(Some of the batches have different levels of DNA plasmid contamination, which are correlated to higher adverse events)
Sadly, there is DNA plasmid contamination in the LNPs for the Pfizer and Moderna c@vid “vaccines”, and this means, the charge must be different, because DNA plasmid has a high negative charge.
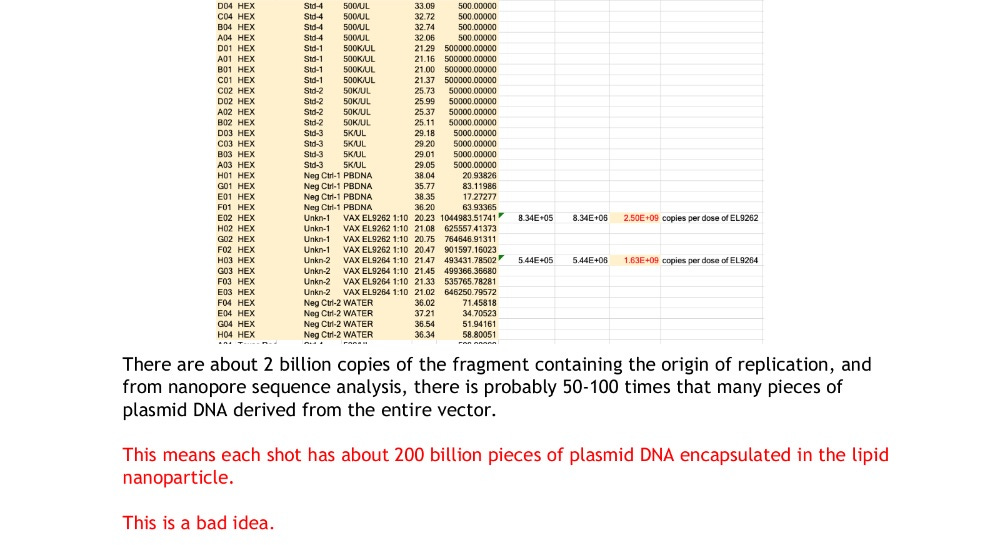
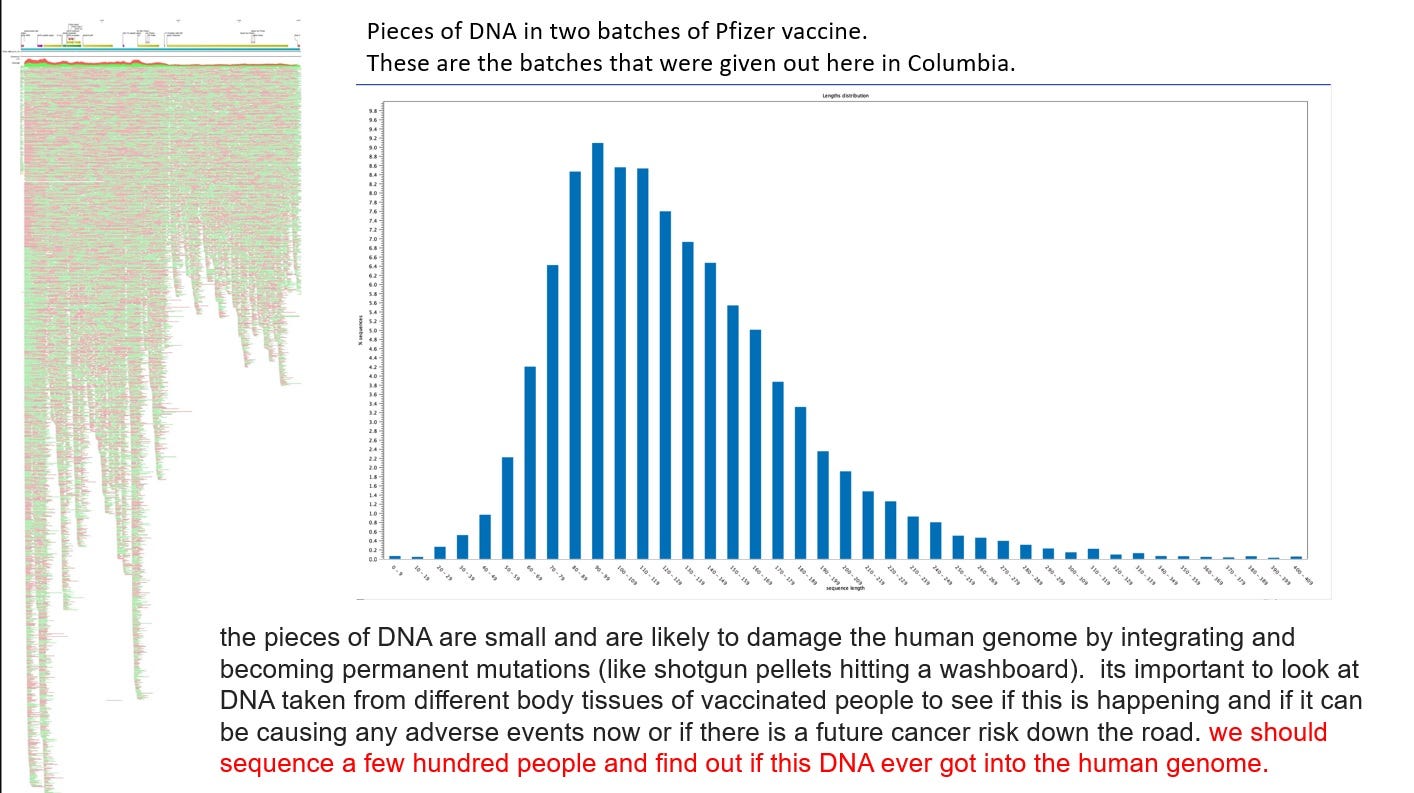
If we do simple math, based on Dr. Buckhaults and Kevin McKernan’s findings, there should be about 4000 on average pieces of DNA plasmid in each LNP now, if they were evenly distributed, with an average base pair size of anywhere between 100 to 120, although, sizes were found much higher and lower.
Due to its negative charge, the lipid nanoparticle may also engage in electron transfer processes with positively charged biomolecules within the nucleus and nucleoli.
This could result in the reduction of PL lifetime observed if the electron transfer leads to modifications in the electronic structure of the nanoparticle or alterations in its chemical environment.
PL lifetime stands for "photoluminescence lifetime."
It refers to the average time it takes for an excited fluorophore, such as a quantum dot or other fluorescent nanoparticle, to return to its ground state after being excited by photons. This electron transfer could lead to modifications in the electronic structure of the nanoparticle or alterations in its chemical environment. As a result, the time it takes for the nanoparticle to emit fluorescence decreases, leading to a reduction in its photoluminescence lifetime.
Now, we are going to talk about the BINDING and interactions occurring within the nucleus, as observed, in this study.
The QDs in the study, BOUND themselves to the histones in the nucleus. This was due to strong electrostatic interactions between the highly positive and negative charges.
The binding of negatively charged QDs to core histones is attributed to the positive charge of core histones, which are approximately 30–40% positively charged due to the presence of lysine and arginine amino acids on their N-termini.
Nuclear lysate also shows strong binding of QDs, due to the presence of other macromolecules with similar properties or changes in histone/QD affinity during protein purification steps.
Binding with the histones means the functionality of the histones would change. You can't just change histone functionality without consequences.
You could ask, why the highly negatively charged LNP did not go to the ribosome to make a protein? The ribosome has a preference for a certain size, and a certain charge on the LNP. It is not a fan of that highly negative charge. Also, the LNP is going to want to go to the nuclear pore and connect with the positively charged histones inside due to electrostatic interactions, because that is what charged particles do (if the LNP has high negative charge).
Interactions with the human body and potential impacts beyond histones:
Both LNPs and QDs have the potential to interact with DNA. LNPs containing genetic material (plasmid DNA or RNA) may deliver these molecules into cells, where they could integrate into the genome or influence gene expression.
LNPs or QDs interfere with P53 function, it could potentially lead to uncontrolled cell growth and cancer formation.
The effects of LNPs and QDs on cells could be transient or ongoing, depending on factors such as nanoparticle stability, cellular uptake and clearance mechanisms, and the persistence of nanoparticle-induced changes in cellular function.
Some effects may be reversible once nanoparticle exposure ceases, while others could have long-term consequences.
LNPs or QDs may interact with cells within the tumor microenvironment, including immune cells, fibroblasts, and endothelial cells.
If LNPs or QDs interfere with DNA repair mechanisms or induce DNA damage within tumor cells, it could potentially contribute to tumor suppression or promote genetic instability and tumor progression.
LNPs Could interact with Pold1 (DNA Polymerase Delta 1), a protein involved in DNA replication and repair processes.
If they enter the nucleus of the cell where Pold1 is primarily located.
Once inside the nucleus, the nucleic acids carried by the LNPs may encounter Pold1 during DNA replication or repair processes.
Pold1 plays a crucial role in maintaining genome stability by accurately replicating DNA and participating in DNA damage repair pathways. Disruption of Pold1 function could lead to the accumulation of mutations, chromosomal abnormalities, or cell death, depending on the extent of DNA damage and the cell's ability to repair it.
COLON CANCER
Pold1 mutations or dysregulation have been implicated in various cancers, including colorectal cancer.
Alterations in Pold1 expression or activity can contribute to tumor initiation, progression, or treatment resistance by promoting genomic instability or affecting DNA repair pathways.
In the study involving QDs and histones, they play a crucial role in gene regulation by controlling the accessibility of DNA to transcription factors and other regulatory proteins. Disruption of histones could lead to dysregulation of gene expression, potentially resulting in abnormal cell function or development.
This could render DNA more vulnerable to damage from environmental factors such as radiation or chemical toxins, increasing the risk of mutations and genomic instability.
This could also lead to cellular dysfunction, such as impaired cell division, DNA repair defects, or aberrant cell signaling.
Histone modifications, such as methylation, acetylation, and phosphorylation, contribute to the epigenetic regulation of gene expression patterns.
Disruption of histones could alter the patterns of histone modifications, resulting in aberrant epigenetic regulation and potentially contributing to diseases like cancer or developmental disorders.
Epigenetic modifications of histones in cancer
Histones play a role in regulating the cell cycle by coordinating chromatin condensation and DNA replication. Disruption of histones could disrupt these processes, leading to abnormalities in cell cycle progression, cell proliferation, and potentially contributing to tumorigenesis or other pathological conditions.
Point of fact, if the study on QDs shows that particles are entering the nucleus based on charge alone, if an LNP has a high negative charge to it, it can do the same, enter the nucleus, and latch on to the LNP.
To jump off the other study regarding positively charged lipids creating adducts with the DNA plasmid inside the LNP, those adducts with the DNA could also enter the nucleus via that NLS mechanism, and with the positively charged lipids, impact the DNA inside the nucleus.
The LNP can enter the nucleus, as its whole self, if it is within a certain size to get in through that nuclear pore, and if it has a high negative charge on it, and interact with histones, and bind to them, among other things.
Even if it just had a charge on it, no DNA, no SV40—nada.




Excellent work
As always
We miss you on X. You were right all along. Did you see this?
https://x.com/onyxnz/status/1767612649662288332?s=20