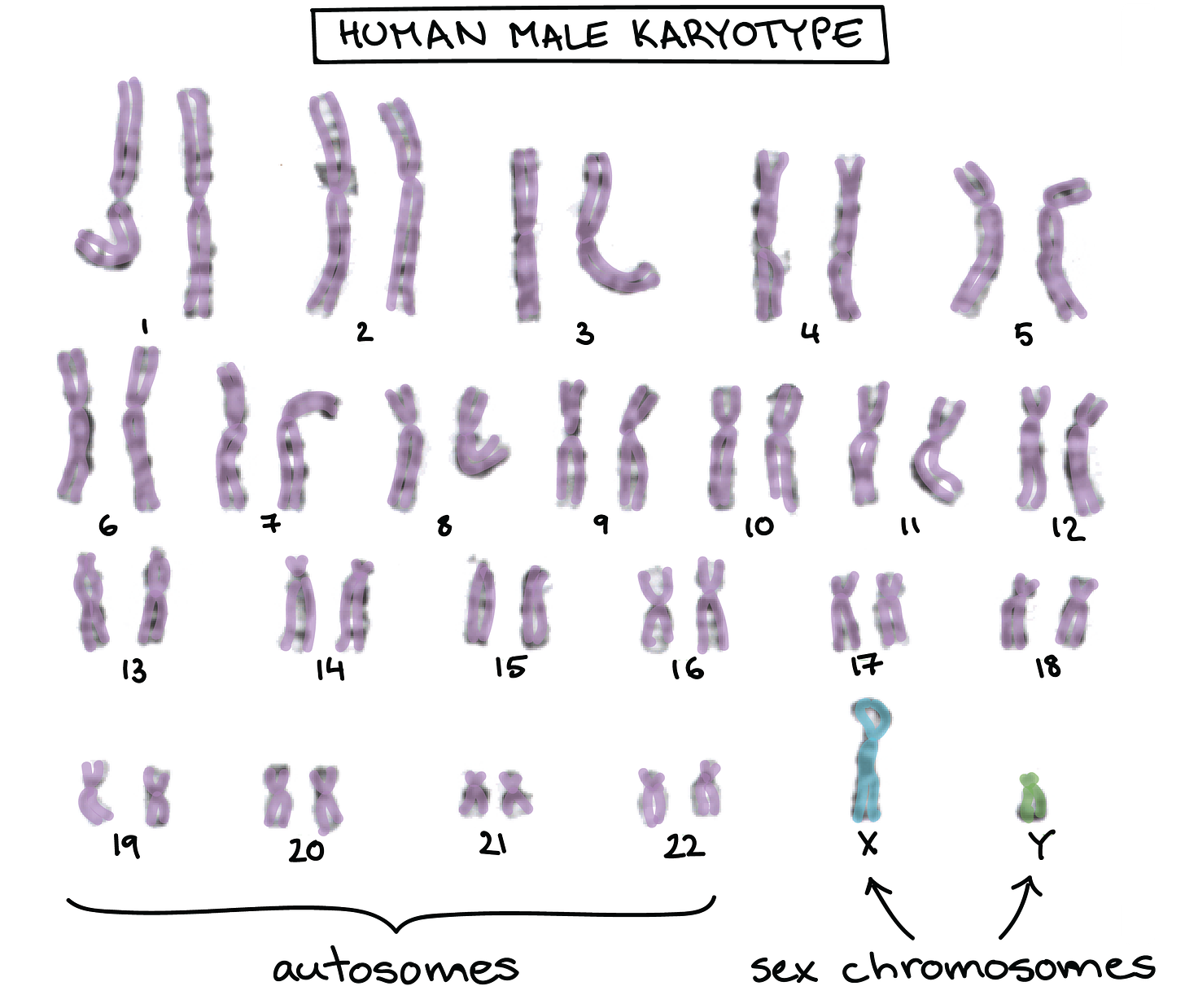The Reasons Why Women Suffer More Adverse Events From Vaccines and mRNA Injections Compared to Men
(This is a repost from my former Substack, from Monday, July 3rd, 2023 at 8:45 AM)
Even at a 1:1 uptake ratio (men to women), women would still suffer more.
The Reasons Why Women Suffer More Adverse Events From Vaccines and mRNA Injections Compared to Men
Even at a 1:1 uptake ratio (men to women), women would still suffer more.
JUL 2
Thanks for reading Christie’s Substack! Subscribe for free to receive new posts and support my work.
(updated cGAS STING and genomics sections at the end)
Copyright Policy of Substack: https://substack.com/dispute
There have been questions regarding the prevalence of vaccine injury, including attenuated vaccines and the recent mRNA injections, which are a transfection event (the use of messenger RNA encased in a lipid nanoparticle to use the body’s own “machinery” to generate a spike protein), and the reasons why women are more greatly impacted with adverse events compared to men.
This Substack will go over as many of the reasons why, that even if the uptake of vaccines, or the injections of mRNA was at a 1;1 ratio for women versus men, women would still by a far greater amount, suffer from increased adverse and severe adverse events, in most cases.
TL;DR (Too long; Didn’t Read: the immune system, hormones, and genes—XX versus XY--are the reasons.)
This is going to be long, because we cannot assume everyone is jumping off from the same starting point of knowledge. Therefore, we must give a background of the female reproductive system, physiology, and immunology--as it relates to women.
(This is mostly for the guys out there.)Developmental Physiological (Hormones) and Immunological Changes Throughout the Lifespan of Women
Women, as many know, go through several changes from birth to death.
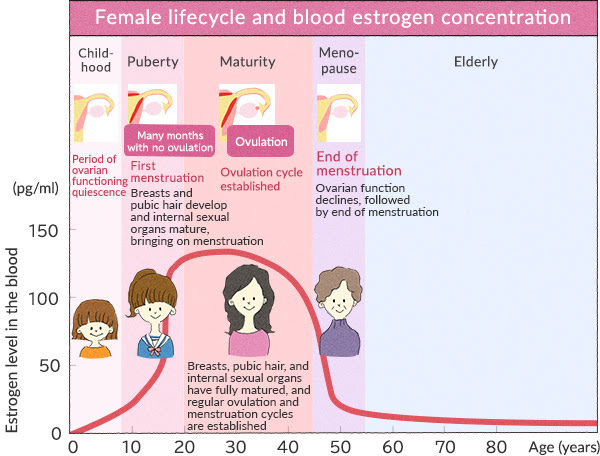
Before the onset of menarche (the first occurrence of menstruation), there are significant differences in the hormonal and immune system profiles of young adolescent girls compared to women who have already gone through menarche (menstruation).
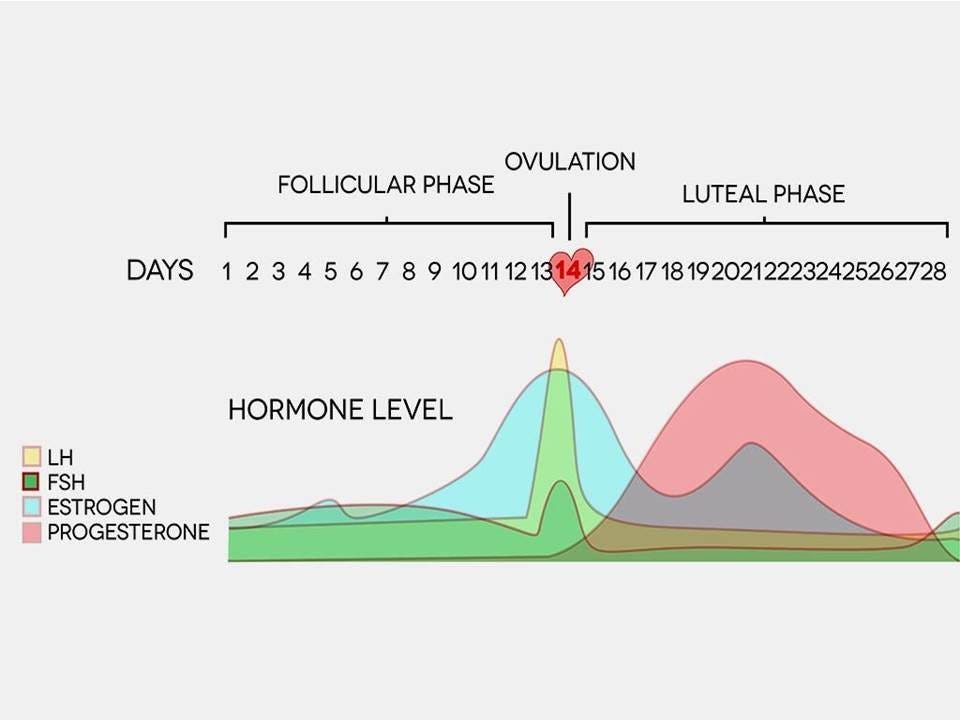
Ovaries and Hormones
The first cycle of a woman (or girl’s life) is called the pre-menarche stage.
Before menarche, the ovaries are not yet fully developed, and there is limited production of reproductive hormones such as estrogen and progesterone. The levels of these hormones are relatively low compared to post-menarche women. This is important to note, for the hormone estrogen is one we will focus on quite a bit in this presentation.
For those who really do not have a clue, menarche is the term used to describe the onset of menstruation in young girls, marking the beginning of their reproductive years. It is a significant milestone in the physical and hormonal development of girls as they transition into young women. This typically happens between the ages of 9 and 15. The mean onset age of menarche is 12, although this varies quite a bit due to genetics, environment, stress, hormone exposure, nutrition, and physiological factors. After menarche, this marks the beginning of the post-menarche stage. During this time, the ovaries undergo maturation and hormonal changes. The menstrual cycle begins, and there is a cyclic release of estrogen and progesterone, which regulate various physiological processes in the body, including immune responses. Another important thing to note about the pre-menarche stage is the immune system is still developing and is relatively immature. The influence of reproductive hormones on immune function is minimal. This means there will most likely be a lower immune response to various stimuli. This is important when we look at adverse events.
The time after this is called post-menarche. After menarche, the cyclic fluctuations of estrogen and progesterone during the menstrual cycle can affect the immune system. Estrogen has been associated with enhancing immune responses, promoting antibody production, and potentially exerting anti-inflammatory effects. Progesterone, on the other hand, can have immunomodulatory effects and may dampen certain immune responses.
This is all going to be coming back into play when we discuss vaccines, mRNA, and adverse events. For those of you who are doctors, clots should come to your minds immediately.The next stage in a woman’s life is perimenopause, and then followed by menopause.
Perimenopause is the timeframe leading up to menopause when a woman's reproductive hormone levels start to decline. It typically begins in the late 30s or 40s, but the timing can vary. During perimenopause, hormone levels, particularly estrogen and progesterone, become more erratic and eventually decrease. This also marks unique changes which occur in a woman’s immune system. Estrogen plays a crucial role in modulating the immune system. As estrogen levels fluctuate and decline during perimenopause, it can affect immune responses. Estrogen has been associated with enhancing immune function, promoting antibody production, and exerting anti-inflammatory effects. The decline in estrogen during perimenopause can potentially lead to changes in immune responses and may contribute to an increased risk of certain autoimmune diseases and infections. This information should get your brain light bulb glowing at full charge. I think you know what is coming.
Menopause marks the end of a woman's reproductive years and is defined as the absence of menstrual periods for 12 consecutive months. It usually occurs around the age of 50, but again, individual experiences may vary. There are significant hormonal changes occurring during this time, which also impact the immune system. In addition to that, there are immune system changes which are occurring for both men and women, which mark decreases in certain immune system responses, due to changes in micro RNA (not due to the mRNA we are talking about in the jabs, we will get there). During menopause, there is a significant decrease in the production of estrogen and progesterone by the ovaries. The hormonal fluctuations and eventual decline can have wide-ranging effects on various bodily systems, including the immune system. Estrogen has immunomodulatory effects, and its decline during menopause can result in immune system changes. Women in menopause may experience alterations in immune function, which can affect their susceptibility to infections, autoimmune diseases, and inflammatory conditions. The decline in estrogen levels may contribute to a chronic low-grade inflammatory state and potential immune dysregulation.Immune System Changes Which Occur During a Women’s Menstrual Cycle
Not only are immune system and inflammatory changes occurring throughout a woman’s lifetime, but they are also occurring during her menstrual cycle. During the menstrual cycle, the levels of hormones such as estrogen and progesterone rise and fall in a cyclical pattern. These hormonal changes can impact the immune response in several ways.
Inflammation
Estrogen, which is at its lowest levels during menstruation, has anti-inflammatory properties. As a result, inflammation may be slightly reduced during this phase of the cycle. This means women will potentially experience less inflammation after receiving a vaccine, or mRNA jab. One cannot be certain.
Immune response
In women, immune response may be enhanced during the pre-ovulation phase of the menstrual cycle, known as the follicular phase. This phase is characterized by rising estrogen levels, which can stimulate certain immune cells and increase their activity. Conversely, during the luteal phase, which occurs after ovulation, progesterone levels increase, and this hormone may have immunosuppressive effects, potentially dampening the immune response.
Susceptibility to infection
The fluctuations in hormone levels during the menstrual cycle can affect the body's susceptibility to certain infections. For example, some women may experience a higher risk of urinary tract infections (UTIs) during certain phases of their menstrual cycle.
Hormonal Influence on the Immune System and Unique Responses to Traditional Attenuated Vaccines in Women
There are several differences in the immune system responses when men and women are given attenuated vaccines.
Antibody production
Studies reflect women produce higher levels of antibodies in response to vaccination compared to men. This enhanced antibody response is believed to be influenced by sex hormones, particularly estrogen, which can amplify the immune response. Consequently, women often exhibit higher antibody titers after vaccination.
Vaccine efficacy
Due to the potentially higher antibody response, studies show evidence that women have slightly higher vaccine efficacy compared to men. For example, in influenza vaccination, women have demonstrated a better protective response against certain strains of the virus.
Adverse events
Adverse events to vaccines can occur in both men and women but may differ in their prevalence. Some studies show that women may experience more frequent and stronger local and systemic reactions following vaccination. These are related to hormonal and genetic factors that can influence immune responses.
(We will touch base on what it means for women and genetic mutation potential and immunological differences due to having more genes, XX versus XY.)
Autoimmune diseases
Women generally have a higher incidence of autoimmune diseases compared to men. This disparity impacts how the immune system responds to vaccines in individuals with pre-existing autoimmune conditions.Genetic differences and more immunology explored
Sex differences in uptake and T Cells
Studies conducted on both humans and animals, mostly focusing on young adults, have found that innate immune responses vary between males and females. In young adult mice, females have shown higher activity in certain immune cells and molecules involved in recognizing pathogens, producing inflammation, presenting antigens, and engulfing foreign substances. It is unclear whether these differences persist in older individuals, but some data suggest that elevated inflammation in females compared to males may continue into old age.
Females also tend to exhibit stronger immune responses in terms of both antibody production and cellular immune responses compared to males. This means that females generally have higher levels of antibodies and a greater immune reaction when exposed to viruses, vaccines, or infections.
Men reportedly have lower absolute CD3+ T-cell counts, absolute numbers of CD4+ T cells, CD4+-to-CD8+ T-cell ratios, and helper T-cell type 1 (Th1) responses. This means that women will have a more robust response, and potential adverse and sever adverse reaction compared to men, when the immune system is at play.
Among older individuals, there is limited data indicating that age-related decline in adaptive immune responses, including T and B cell numbers and cytokine production, may be more significant in males compared to females.
Females report more adverse reactions to vaccination and have more concerns about vaccine safety and efficacy than males, which contributes to the differences in the uptake of influenza vaccines among aged males and females.
Adverse Reactions to Vaccines are Greater in Aged Females Than in Males
When it comes to older individuals, females consistently report more adverse reactions compared to males after receiving vaccines for seasonal and pandemic influenza, pneumococcal diseases, herpes zoster (shingles), tetanus, and pertussis (whooping cough). Both males and females experience similar types of reactions, but the proportion of females reporting these reactions, such as pain, redness, swelling at the injection site, as well as muscle or joint pain, headache, fever, and hypersensitivity reactions, is consistently higher than in males.
In terms of antibody responses, which are important for vaccine effectiveness, there are differences between males and females among older individuals. In general, aged males and females have lower antibody responses to vaccines compared to younger adults. Aged females consistently show higher antibody responses to influenza vaccines compared to males. This led to the development of a high-dose influenza vaccine specifically for individuals aged 65 and older, regardless of sex, to address the lower antibody production in this age group. The differences in antibody responses between sexes are evident for various influenza strains, where older females have significantly higher antibody levels compared to males.Your chrommies are short dudes—women have more genes—leading to higher immune system responses, higher autoimmunity disease, vaccine adverse events, and genetic mutations
Women have a higher risk of developing autoimmune diseases compared to men, with up to four times greater likelihood. Several factors include sex hormones, the X chromosome, microchimerism (presence of cells from another individual), environmental factors, and the microbiome.
Microchimerism refers to the presence of a small number of cells or DNA from another individual within an individual's body. It occurs when cells or genetic material from a different individual, typically from a mother to her child or vice versa, enter the bloodstream and become integrated into the recipient's tissues.
During pregnancy, fetal cells can cross the placenta and enter the mother's bloodstream, leading to maternal microchimerism. Similarly, maternal cells can also cross into the fetus and contribute to fetal microchimerism. These cells can persist in the recipient's body for many years, even decades, after pregnancy.
Microchimerism can also occur through other mechanisms, such as blood transfusions or organ transplantation, where foreign cells or DNA are introduced into an individual's body. The presence of microchimerism is a natural and relatively common occurrence, although the extent and duration of microchimeric cells can vary among individuals. It has been suggested that microchimerism may have potential implications for health and disease, including autoimmune disorders, cancer, and tissue repair
During the postpartum period, when antibody levels are elevated, there is a peak in autoimmune diseases. Third, there is a relationship between the number of children a woman has given birth to (parity) and both antibody levels and the risk of autoimmune disease. Finally, there are plausible biological mechanisms, such as the activation of B cells by T cells or a protein called VGLL3, that can explain the association between antibodies and autoimmunity.From an evolutionary perspective, the higher levels of antibodies in women may be advantageous for protecting their offspring from infections. However, this may also make women more susceptible to developing autoimmune diseases.
”Sex SteroidsThe prevailing hypothesis for immunological differences between the sexes is that sex steroids, particularly testosterone, estradiol, and progesterone, influence the functioning of immune cells. Sex steroids alter the functioning of immune cells by binding to specific receptors, which are expressed in various lymphoid tissue cells as well as in circulating lymphocytes, macrophages, and DCs.
The binding of sex steroids to their respective steroid receptors directly influences cell signaling pathways, including NF-κB, cJun, and IRF1, resulting in differential production of cytokines and chemokines.
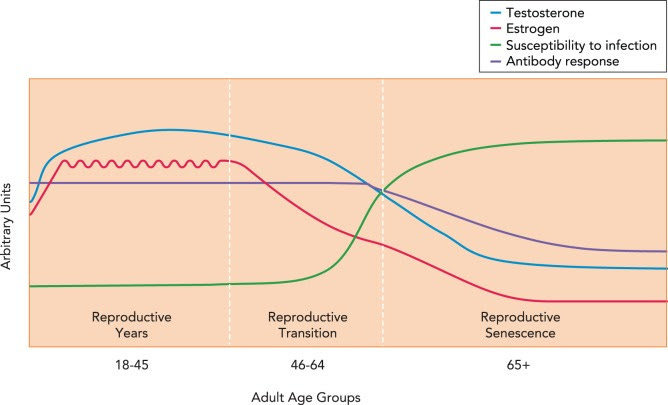
[This means women are more susceptible to cytokine storms, mast cell disease concerns, and other issues with the chemokines and cytokines.]With age, the hormonal milieu dramatically changes as ovarian function in females and testicular production of sex steroids in males decline. The hormonal changes associated with menopause in females are dramatic and relatively abrupt, since ovarian production of estradiol declines and progesterone production is reduced to that which is synthesized by the adrenal glands. In males, the reduction in testosterone production is more gradual.
The reduction in sex steroid concentrations and sex steroid receptor signaling with age likely contributes to age-associated dysregulation of immune function (FIGURE 1) (45). This has been directly studied in females pre- and postmenopause, with menopause (either naturally occurring or surgically induced) resulting in lower numbers of B and T cells and greater concentrations of proinflammatory cytokines (e.g., IL-1β, IL-6, and TNF-α).Genetic and Epigenetic Regulation
In addition to hormonal influences, genetic and epigenetic factors contribute to sex-based differences in an immune response to vaccination.
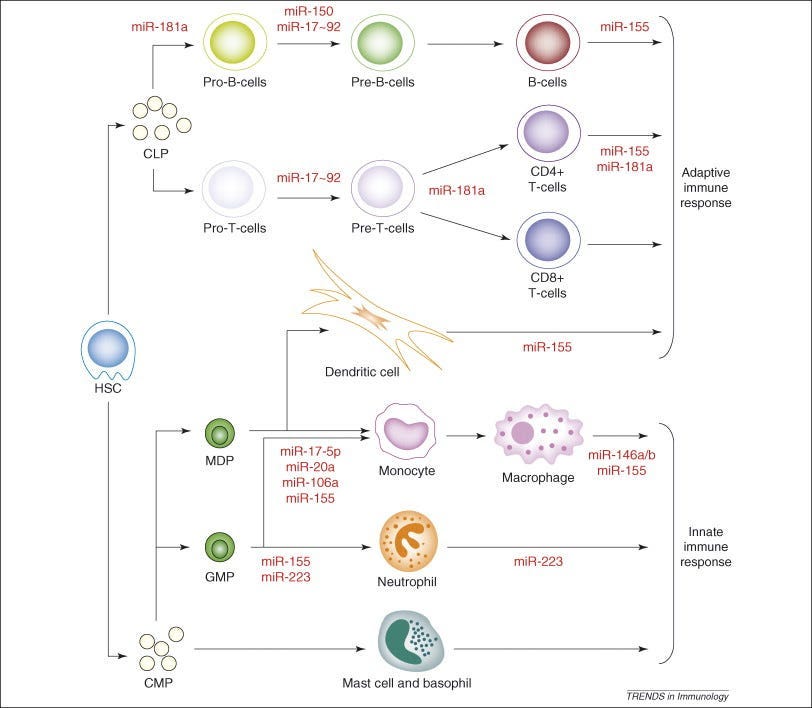
Sex-based differences in responses to vaccines are observed before puberty, during reproductive years, and after reproductive senescence, suggesting a role for factors other than sex steroids. A large number of immune-related genes encoding proteins are located on the X chromosome (38). A number of critical transcriptional and translational control effectors that function downstream of activated cytokine receptors are also encoded on the X chromosome. Given that males are XY and females are XX, any damaging mutations or polymorphisms to X-linked genes are more likely to have an immune consequence in males compared with females. The combined effects of hormones influencing the epigenetic regulation of gene expression, and gene composition on the X chromosome potentially differing between XX females and XY males, might determine an immune response to vaccination.The expression of X-linked genes may be affected by X-linked micro-RNAs (miRNAs), which are small noncoding RNAs that regulate gene expression at a posttranscriptional level and play a role in maintaining immunological homeostasis.
There are disproportionately more miRNAs located on the X chromosome than on any autosomal chromosome.[Females have TWO X chommies, males have only one, meaning, we are at higher risk for all of the adverse events, the cancers, the mutations, the autoimmune disorders, the gut microbiome disorders, etc.)
The X chromosome contains 10% of the ∼800 miRNAs in the genome, whereas the Y chromosome contains only 2 miRNAs.
Lastly, polymorphisms in sex chromosomal and autosomal genes that encode for immunological proteins can contribute to sex differences in immune responses and antibody responses to vaccination.”
Autoimmune disorders and women
”The larger number of genes originating from the X chromosome creates a far greater possibility of a larger number of mutations occurring. This puts women at a greater risk for the development of autoimmune diseases solely due to women having two X chromosomes, whereas men possess only one. The presence of two X chromosomes essentially creates a 'double dose' of genes present on the X chromosome and because of this, predisposes the female to autoimmunity. “
When speaking to the presence of more genes than men, this means that women are more susceptible not only to autoimmune diseases, but adverse events from a vaccine or mRNA product because there is a gargantuan difference between the number of men who have auto immune diseases versus women. Women to men, ratio 4:1, experience Multiple Sclerosis.
Multiple women do not even know they have an auto immmune disorder, and if a woman has a disorder, when getting a vaccination or mRNA injection, this could set her up for higher risk of adverse and severe adverse events.
This really sucks typing this as a woman.
Why Nearly 80 Percent of Autoimmune Sufferers Are Female
”Melanie See’s first bout of odd symptoms began in 2005. Suddenly she started sweating a lot. She rapidly lost 10 pounds. She got dizzy walking from the bedroom to the couch. She started lactating even though she was not nursing a baby. After a slew of laboratory tests, See, then 45, was diagnosed with Graves’ disease, an autoimmune disorder that makes thyroid hormones surge.Three years later, when See’s symptoms from Graves’ were under control with medication, her health took another rapid downturn. She lost more weight. She felt extremely tired. Her doctors diagnosed her with celiac disease, another autoimmune disease, which in affected people is set off by eating foods with gluten. Then, in 2015, See, who lives in Chapel Hill, N.C., began experiencing terrible digestive symptoms and muscle pain. This time her doctors were stumped. “Initial diagnoses were all over the place—vasculitis, lupus, I can’t remember what all,” See says. “My blood work showed something was going on, as did the muscle biopsy I had in June 2016, but I didn’t fit into any particular box.”
After many tests, See was diagnosed with yet a third autoimmune illness: mixed connective tissue disease, a rare ailment that shares some features of lupus.”
”Women account for an estimated—and astonishing—78 percent of people who have these disorders, which include See’s afflictions, as well as lupus, multiple sclerosis, rheumatoid arthritis, and other illnesses in which the body’s immune system mistakenly attacks its own cells and tissues. Autoimmune diseases are now the fifth-leading cause of death in women younger than 65.”
”In 1997 a consortium of Finnish and German scientists discovered a gene that plays a crucial part in autoimmunity. This gene, which they named AIRE, for “autoimmune regulator,” is expressed by cells in the thymus, an organ that makes T cells. AIRE ensures that key body proteins are shown to developing T cells, and these encounters teach T cells that the proteins are friends, not foes. Also thanks in part to AIRE, T cells that start attacking these friendly proteins are destroyed in the thymus before they can be released into the rest of the body, where they could do damage.”
cGAS STING Pathway and how this is Different in Women, Compared to Men, and how this impacts autoimmune disease and cancer, for women.
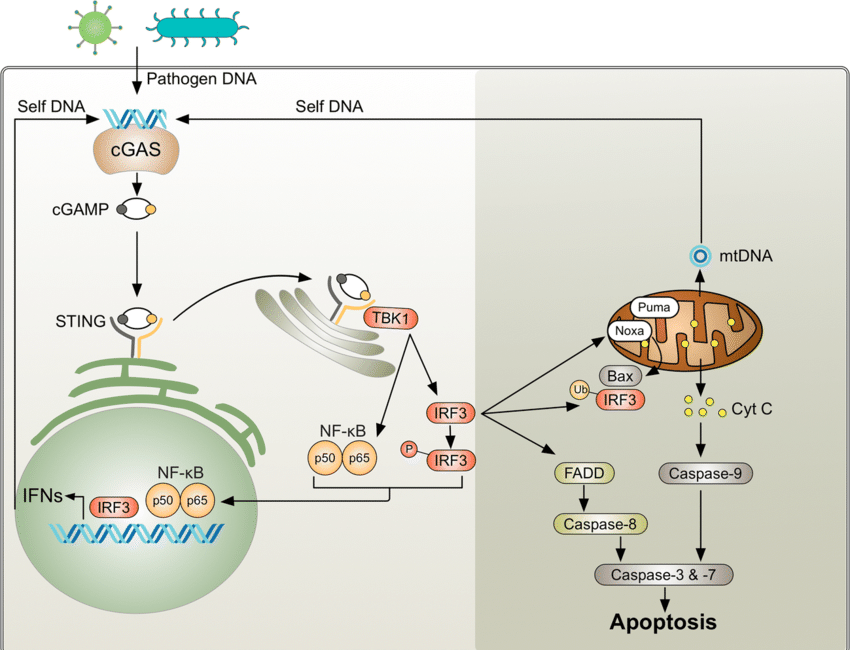
Start by reading this whopper on cGAS STING and how it is activated by mRNA “vaccines” to understand. I will come back ad update with a layman’s version.Now, let’s talk about why cGAS STING is different in women, some examples, and how this impacts autoimmune disease and cancer, for WOMEN:
cGAS-STING pathway, a crucial component of the innate immune system responsible for detecting cytosolic DNA and triggering an immune response, is generally the same in both women and men.
cGAS STING can be activated by viruses, bacteria, DNA, including OUR DNA, DNA plasmid pieces (like those found as contamination in the current mRNA “vaccines”. and the SPIKE protein (as well as COVID). But pieces of DNA plasmid will bind readily to that.
However, there can be differences in the regulation and activity of this pathway due to hormonal, genetic, and epigenetic factors that differ between the sexes.
Sex hormones such as estrogen and testosterone can modulate immune responses. Estrogen, has been shown to influence the expression of various immune-related genes, potentially affecting the cGAS-STING pathway. Women, with higher levels of estrogen, might have different regulatory mechanisms influencing the pathway compared to men—meaning, a stronger immune response and stronger reaction through cGAS STING.
Women generally have a more robust immune response compared to men, which can be partly attributed to differences in immune cell types and their activities.
This heightened immune response in women might influence the activity and outcomes of the cGAS-STING pathway
In women, genes located on the X chromosome might exhibit differential expression due to X chromosome inactivation.
For instance, genes related to immune function, including those involved in the cGAS-STING pathway, may be expressed at higher levels in women compared to men due to escape from X chromosome inactivation.
Estrogen receptors, which are present in higher concentrations in women, can directly influence the expression of genes involved in immune responses.
Estrogen signaling pathways can enhance the expression of certain components of the cGAS-STING pathway.
TLR7 is an innate immune receptor located on the X chromosome and is involved in recognizing single-stranded RNA viruses.
Studies have shown that TLR7 can escape X chromosome inactivation in women, leading to higher expression levels compared to men.
TLR7 activation can trigger downstream signaling pathways, potentially impacting the activity of the cGAS-STING pathway. TLR7 activation has been implicated in the pathogenesis of autoimmune conditions like systemic lupus erythematosus.D40L, also known as CD154, is another gene located on the X chromosome that plays a crucial role in adaptive immune responses by providing co-stimulatory signals to antigen-presenting cells.
CD40L expression has been found to escape X chromosome inactivation in women, resulting in higher levels compared to men.
CD40-CD40L interactions can modulate immune responses, which may indirectly affect the activity of the cGAS-STING pathway.
CD40L interactions have been associated with autoimmune diseases such as systemic sclerosis and rheumatoid arthritis. Therefore, increased CD40L expression could potentially contribute to the development or exacerbation of autoimmune diseases in women.
FOXP3 is a transcription factor essential for the development and function of regulatory T cells (Tregs), which play a critical role in maintaining immune homeostasis and preventing autoimmunity.
Mutations in the FOXP3 gene can lead to immune dysregulation and autoimmune diseases.
As FOXP3 is located on the X chromosome, its expression may vary between men and women due to X chromosome inactivation, influencing immune responses mediated by Tregs and impacting the cGAS-STING pathway indirectly.
Dysregulation of FOXP3 expression or function has been implicated in autoimmune diseases such as autoimmune polyendocrine syndrome and type 1 diabetes.
Monoamine oxidase A (MAOA) and monoamine oxidase B (MAOB) are enzymes involved in the metabolism of neurotransmitters such as serotonin, dopamine, and norepinephrine.
Variations in the activity of MAOA and MAOB have been associated with psychiatric disorders and neuroinflammatory conditions.
Both MAOA and MAOB are located on the X chromosome, and their expression may differ between men and women due to X chromosome inactivation, potentially affecting neuroimmune interactions that could modulate the cGAS-STING pathway.
Variations in MAOA and MAOB expression due to X chromosome inactivation might influence neuroinflammatory processes, potentially contributing to the development or progression of CNS-related autoimmune diseases.
The way this would happen, is very context dependent and other genetic and environmental factors will come into play.
The science:
Estrogen Receptors and cGAS-STING Pathway:
Estrogen receptors, particularly ERα and ERβ, are expressed at higher levels in women and can directly influence immune responses.
Estrogen signaling pathways have been shown to upregulate the expression of certain components of the cGAS-STING pathway, such as STING (Stimulator of Interferon Genes) and IRF3 (Interferon Regulatory Factor 3), through transcriptional regulation.
For example, estrogen-activated ERα can bind to estrogen response elements (EREs) in the promoter regions of genes involved in the cGAS-STING pathway, leading to enhanced gene expression.
This estrogen-mediated upregulation of cGAS-STING pathway components can result in heightened immune responses to viral infections and other cytosolic DNA triggers in women compared to men.
MicroRNA Regulation and cGAS-STING Pathway:
MicroRNAs (miRNAs) are small non-coding RNAs that post-transcriptionally regulate gene expression by binding to the 3' untranslated regions (UTRs) of target mRNAs.
Sex-specific expression patterns of miRNAs can fine-tune the expression of key genes in the cGAS-STING pathway differently in men and women.
For instance, miRNAs such as miR-21 and miR-155 have been shown to target and regulate components of the cGAS-STING pathway, including cGAS and STING, thereby modulating its activity.
Differences in the expression levels or activities of these miRNAs between sexes can influence the responsiveness of the cGAS-STING pathway to cytosolic DNA stimuli and subsequent immune responses.
DNA Methylation and Histone Modifications and cGAS-STING Pathway:
Epigenetic modifications such as DNA methylation and histone modifications play crucial roles in regulating gene expression.
Sex-specific patterns of DNA methylation and histone modifications can affect the accessibility of genes involved in immune responses, including those in the cGAS-STING pathway.
For example, differential DNA methylation of promoter regions or enhancers of cGAS and STING genes may modulate their expression levels between sexes.
Similarly, histone modifications such as acetylation, methylation, and phosphorylation can influence the chromatin structure and accessibility of cGAS-STING pathway genes, thereby impacting its activity.
Sex-specific epigenetic regulation of the cGAS-STING pathway genes can contribute to variations in immune responses and susceptibility to infections or autoimmune diseases between men and women.
Conclusion
Women suffer from vaccine reactions at a much higher rate than men. It would ont matter if uptake was equal, or if even more men received more vaccinations than women. it would not matter if more men received more mRNA injections than women. The outcome would be the same: women would suffer more due to genetic differences, sex hormone differences, immunological differences, and evolutionary biologicals differences.
References
Angum, F., Khan, T., Kaler, J., Siddiqui, L., & Hussain, A. (2020). The Prevalence of Autoimmune Disorders in Women: A Narrative Review. Cureus, 12(5), e8094. https://doi.org/10.7759/cureus.8094
Carrero, J. J., Stenvinkel, P., Fellström, B., Qureshi, A. R., Lamb, K., Heimbürger, O., & Barany, P. (2008). Telomere attrition is associated with inflammation, low fetuin-A levels and high mortality in prevalent haemodialysis patients. Journal of Internal Medicine, 263(3), 302-312. doi: 10.1111/j.1365-2796.2007.01899.x
Cook, I. F. (2008). Sexual dimorphism of humoral immunity with human vaccines. Vaccine, 26(29-30), 3551-3555. doi: 10.1016/j.vaccine.2008.04.053
Cutolo, M., & Sulli, A. (2006). Estrogen, cytokines, and immunity. Autoimmunity Reviews, 5(2), 136-140. doi: 10.1016/j.autrev.2005.04.008
Engler, R. J., Nelson, M. R., Klote, M. M., VanRaden, M. J., Huang, C. Y., Cox, N. J.,... & Cox, A. (2008). Half- vs full-dose trivalent inactivated influenza vaccine (2004-2005): age, dose, and sex effects on immune responses. Archives of Internal Medicine, 168(22), 2405-2414. doi: 10.1001/archinte.168.22.2405
Fink, A. L., & Klein, S. L. (2015). Sex and Gender Impact Immune Responses to Vaccines Among the Elderly. Physiology (Bethesda, Md.), 30(6), 408–416. https://doi.org/10.1152/physiol.00035.2015
Fire A. et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature.1998;391:806-811
Furman, D., Hejblum, B. P., Simon, N., Jojic, V., Dekker, C. L., Thiébaut, R.,... & Davis, M. M. (2014). Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proceedings of the National Academy of Sciences, 111(2), 869-874. doi: 10.1073/pnas.1319240111
Gérard, H. C., & Whittum-Hudson, J. A. (2017). Sex hormones and gender influence the host response to infectious agents. Journal of Autoimmunity, 82, 60-75. doi: 10.1016/j.jaut.2017.05.013
Giefing-Kröll, C., Berger, P., & Lepperdinger, G. (2015). Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell, 14(3), 309-321. doi: 10.1111/acel.12326Gleeson, M., Bishop, N. C., Stensel, D. J., Lindley, M. R., Mastana, S. S., & Nimmo, M. A. (2011). The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nature Reviews Immunology, 11(9), 607-615. doi: 10.1038/nri3041
Gubbels Bupp, M. R. (2015). Sex, the aging immune system, and chronic disease. Cellular Immunology, 294(2), 102-110. doi: 10.1016/j.cellimm.2015.02.002
Javierre, Biola M., and Juan Ramón Tejedor. "Regulation of gene expression and RNA splicing by DNA methylation and histone modifications." In Epigenetics and Regeneration. Academic Press, 2019. 209-227
Kim, H. I., Lim, H., & Moon, A. (2018). Sex Differences in Cancer: Epidemiology, Genetics and Therapy. Biomolecules & therapeutics, 26(4), 335–342. https://doi.org/10.4062/biomolther.2018.103
Karpuzoglu, E., & Ahmed, S. A. (2006). Estrogen regulation of nitric oxide and inducible nitric oxide synthase (iNOS) in immune cells: implications for immunity, autoimmune diseases, and apoptosis. Nitric oxide : biology and chemistry, 15(3), 177–186. https://doi.org/10.1016/j.niox.2006.03.009
Klein, S. L., & Flanagan, K. L. (2016). Sex differences in immune responses. Nature Reviews Immunology, 16(10), 626-638. doi: 10.1038/nri.2016.90
Kovats, Susan. "Estrogen receptors regulate an inflammatory pathway of dendritic cell differentiation: mechanisms and implications for immunity." Hormones and Behavior 62.3 (2012): 254-262.
Kronzer, V. L., Bridges, S. L., Jr, & Davis, J. M., 3rd (2020). Why women have more autoimmune diseases than men: An evolutionary perspective. Evolutionary applications, 14(3), 629–633. https://doi.org/10.1111/eva.13167
Lu, Jun, et al. "MicroRNA expression profiles classify human cancers." Nature 435.7043 (2005): 834-838.
Moyer, M. (2021, September 1). Why Nearly 80 Percent of Autoimmune Sufferers Are Female. Scientific American . https://www.scientificamerican.com/article/why-nearly-80-percent-of-autoimmune-sufferers-are-female/
O'Connell, Ryan M., David S. Baltimore, and John U. Jung. "MicroRNAs and herpesviruses: new mechanisms of host–pathogen interactions." Trends in Microbiology 21.10 (2013): 491-497.
Roadmap Epigenomics Consortium, et al. "Integrative analysis of 111 reference human epigenomes." Nature 518.7539 (2015): 317-330.
Straub, R. H. (2007). The complex role of estrogens in inflammation. Endocrine Reviews, 28(5), 521-574. doi: 10.1210/er.2007-0001
Tsitsiou, E., & Lindsay, M. A. (2009). microRNAs and the immune response. Current opinion in pharmacology, 9(4), 514–520. https://doi.org/10.1016/j.coph.2009.05.003