HYPOTHESIS/PROPOSAL: MODIFICATION of EXISTING STUDY: EPIGENETIC CLOCKS as TRACKING MECHANISMS FOR INJURY and DISEASE--WITH or W/OUT INTEGRATION for FINDING CELLULAR and EPIGENTIC AGE and DAMAGE
TRACK INJURIES "BY TIME"?
This is from a tweet I just made—I have no bones in my body that represent brevity.
I need to expand on this in regards to cGAS STING implications of DNA plasmid activation without integration, creating hypermethylation, leading to myocarditis, autoimmune disease, and cancer.
I will expand more here (later). I was going to place this in the paper I have been working on, but it deserves a paper all on its own.
HYPOTHESIS/PROPOSAL: MODIFICATION of EXISTING STUDY: EPIGENETIC CLOCKS as TRACKING MECHANISMS FOR INJURY and DISEASE--WITH or W/OUT INTEGRATION for FINDING CELLULAR and EPIGENETIC AGE and DAMAGE by RNA/DNA/LNP--TRACK INJURIES "BY TIME"?
(Length of Iliad post)
*In cancers, loss of expression of genes occurs about 10 times more frequently by hypermethylation of promoter CpG islands than by mutations.*
This is heavy science. Use your favorite AI model to copy and paste this for a "explain it to me like I am 12" version. Use your Grok, etc.
A longitudinal study was done and submitted in June of 2022 on those with severe covid and those who received vaccination. During this time, COVID was more virulent--produced more disease compared to other strains. Additionally, those used in this study who received vaccination do not appear to have suffered any type of side effect from vaccination that was immediately apparent.
"Longitudinal Study of DNA Methylation and Epigenetic Clocks Prior to and Following Test-Confirmed COVID-19 and mRNA Vaccination"
https://pmc.ncbi.nlm.nih.gov/articles/PMC9203887/
Pang, A. P. S., Higgins-Chen, A. T., Comite, F., Raica, I., Arboleda, C., Went, H., Mendez, T., Schotsaert, M., Dwaraka, V., Smith, R., Levine, M. E., Ndhlovu, L. C., & Corley, M. J. (2022). Longitudinal Study of DNA Methylation and Epigenetic Clocks Prior to and Following Test-Confirmed COVID-19 and mRNA Vaccination. Frontiers in genetics, 13, 819749.
First, a bit about what epigenetic clocks are and how they are useful in studying diseases, like cancer. Second, we are going to look at some highlights form this study (link below) and then we are going to look at other epigenetic "clocks", CpG islands, and some other factors (more are involved with cGAS STING, APOBEC and other factors that are not discussed here)--not just with aging, but autoimmune disease, CANCER, how one might combine this study design with some other factors, and do some different tests to elucidate determinants of disease states.
Epigenetic Clocks and Their Role in Biological Aging
Epigenetic clocks are biomarkers of biological age that rely on DNA methylation patterns to predict an individual’s physiological age based on their epigenetic modifications.
The most well-known epigenetic clocks include the Horvath clock and the Hannum clock, which focus on specific sets of CpG (cytosine-phosphate-guanine) sites across the genome.
These clocks show massive associations with age-related phenotypes, chronic diseases, and mortality risk, reflecting the cumulative impact of various environmental factors, lifestyle choices, and biological stressors on the aging process.
CpG islands (CGI) which appear in segments of our genome that are rich in the C's and G's in our DNA--are regions with high frequency of cytosine and guanine nucleotides, and higher frequency of CpG dinucleotides than the rest of our human genome. CGIs are found in or near the promoter regions of genes. The promoter is where transcription begins.
These can be methylated--hypermethylated. This can happen without integration, and it can happen with what is called TRANSIENT EXPRESSION.
Aberrant methylation of cytosine nucleotides within CGIs can lead to cancer formation.
In cancers, loss of expression of genes occurs about 10 times more frequently by hypermethylation of promoter CpG islands than by mutations.
Mechanisms of Epigenetic Aging: DNA Methylation
DNA methylation is the addition of a methyl group to the cytosine bases of DNA, mostly occurring in CpG dinucleotides--which can regulate gene expression by influencing the accessibility of transcription factors to DNA.
Hypermethylation is an increase in methylation levels at specific genomic regions, like promoter regions of genes. CpG islands have a unique placement when it comes to our promoters in our Genes.
Hypermethylation of promoter regions generally leads to gene silencing, while hypomethylation can activate gene expression. As individuals age, changes in the methylation patterns across the genome, and this can be tracked, and allow for studying aging, but that is not all.
When a promoter region becomes hypermethylated, the gene it regulates can become downregulated or silenced, disrupting normal cellular pathways causing diseases, including cancers, autoimmune disorders, and chronic inflammatory conditions. An example would be hypermethylation of tumor suppressor gene, which could drive oncogenesis (CANCER) by preventing the expression of proteins that would usually inhibit uncontrolled cell growth.
In the first wave which produced more virulence, hypermethylation of genes associated with the immune response may have led to impaired immune function, potentially exacerbating the effects of Sars COv 2 in some people, which manifested as an altered inflammatory response, contributing to the severity of the disease.
But what about the vaccines? We'll get there.
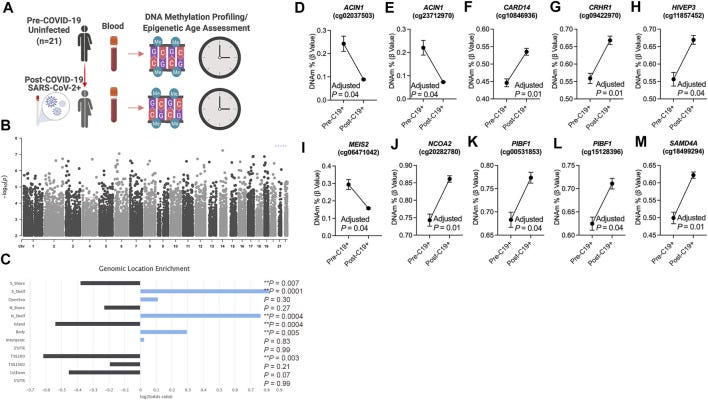
The study--how covid might have impacted epigenetic clocks. The researchers looked at SARS-CoV-2 infection and mRNA COVID-19 vaccinations influence epigenetic clocks—specifically, PCPhenoAge, PCGrimAge, and other measures of biological age—among individuals of varying ages.
(they did not get the right groups of people for the vaccinations--they also MISSED some huge factors)
This study involved examinations of 21 participants aged 18 to 73, with blood-based DNA methylation data collected within a six-month timeframe.
The study showed that specific epigenetic changes, particularly hypermethylation of the CARD14 gene, were associated with alterations in immune cell composition, notably in CD8 T cells. This hypermethylation shows a larger dysregulation of immune function post-infection. Additionally, they identified 756 differentially methylated CpGs related to COVID-19 exposure, with many loci enriched in transcriptional gene sets derived from existing SARS-CoV-2. ACIN1, a gene involved in chromatin condensation during apoptosis, showed a decrease in methylation post-COVID-19.
Methylation changes were linked to 516 protein-coding genes involved in pathways like cellular glucose homeostasis and thyroid hormone signaling.
The study authors were probably also not aware, that the vaccines produced by Pfizer and Moderna contain DNA plasmid contamination.
*******************************************************************
Now, what if we take parts of this study, and then fuse with some other tests? What could we find out?
(this is a social media post--if you are in a lab, maybe you could edit/correct/make some changes on some things. Here we go.)
Temporal Investigation of Vaccine injuries
Epigenetic clocks and molecular scars can help estimate the timing and impact of injury like myocarditis, activation of autoimmune disease, tumor clonal expansion, and other injuries via prolonged genetic/epigenetic dysregulation post-vaccine, particularly if DNA/RNA persists or integrates.
Beyond recognition of epigenetic changes, there may be a way to go back in time, and look and see, when things occurred, molecularly. You could take blood or tissue, without knowing if it came from someone injured, run some tests, and see if there was a hypermethylation, and see if gene expression occurred and what type that would happen in a CpG island adjacent to a promoter region that encodes for--an immune system protein.
In the basic terms, one wonders, if you could focus on Epigenetic and Transcriptomic Analysis.
One could look at DNA methylation analysis by performing bisulfite sequencing on tissues like blood (for systemic immune response markers), heart tissue (for myocarditis markers), and potentially tumor biopsies (if clonal expansion is suspected)--focusing on regions associated with immune response genes, oncogenes, and tumor suppressor genes to track the changes.
Next one would look at targeted methylation panels that focus on known immune-modulated genes and cancer-related genes to determine epigenetic shifts linked myocarditis, autoimmune, or tumorigenesis.
Then one could look at RNA-seq and single-cell RNA-seq and perform gene expression profiling of blood cells, heart tissue, or tumor samples looking at the long-term effects of vaccine-induced inflammation or tumor growth When using single-cell RNA-seq, this can elucidate fine tuned details about cell-specific responses, especially in immune cells (T cells, macrophages) or in expanding tumor clones.
To look at cancer, one could look at clonal tracking and somatic mutations through whole-genome or targeted sequencing:
Detect clonal mutations in key cancer-related genes (KRAS, MYC, or TP53) in tumor tissues or blood (for circulating tumor DNA). Mutations or integration of vaccine-related DNA could indicate clonal expansion triggered post-vaccine. However, this is a very basic approach (the WGS). Single-cell sequencing could track tumor heterogeneity and how it evolved over time. This is all known and basic level.
However, if one looks at methylation states in specific CpG islands, this may assist.
Tracking CpG Methylation in Myocarditis
This involves immune-mediated damage to heart tissue, with immune-related genes (IL-6, TNF-a, TLR pathways) likely undergoing epigenetic modifications.
Even one year after vaccine administration, you could find hypo- or hypermethylation at CpG islands near the promoters of inflammatory cytokine genes in immune cells (like T cells, macrophages) or heart tissue. Hypomethylation (less methylation) in these islands might indicate prolonged gene activation, showing the lingering effects of myocarditis.
Hypermethylation (more methylation) could suggest suppression of regulatory pathways that might normally limit inflammation.
Analyze CpG Methylation in Immune Genes
For myocarditis, look for methylation changes in CpG islands of:
Cytokine genes
IL-6, TNF-a, and IFN-y.
Toll-like receptor genes
TLR3, TLR7, TLR9.
Immune regulatory genes
Genes related to regulatory T cells (Tregs) or macrophage polarization (M1/M2).
***Genes involved in the innate response, like cytokines (TNF-α, IL-6, IL-1β), Toll-like receptors (TLRs), and interferon-related genes, show hypomethylation early in response to infection/injection.
This allows for rapid transcription and the production of pro-inflammatory molecules.
Methylation patterns of innate immune genes change within hours to days of activation, making it possible to detect these events shortly after the immune system is engaged.
****Epigenetic Clocks for Innate Immunity--These clocks might be used to track the rate of cytokine gene methylation changes to estimate how recently the immune system was triggered by the RNA or DNA vaccine.
EXAMPLE: A loss of methylation at the TNF-a promoter suggests rapid immune activation that may have occurred recently.
Over time, there are epigenetic changes in genes controlling T-cell differentiation, antigen presentation, and memory T-cell formation.
Hypomethylation of T-cell genes
In T cells, specific genes associated with T-cell receptor signaling and cytokine production become hypomethylated during the adaptive response.
These changes may last weeks to months after the initial immune challenge, making them suitable for longer-term immune clocks.
Regulatory T cells (Tregs), which express FOXP3, are important for shutting down immune responses and preventing autoimmunity. If FOXP3 is involved, autoimmunity might now be engaged compared to initial response.
This means, you might not even need to know the exact date someone had vaccination to know when injury occurred.
FOXP3 and Regulatory T Cells (Tregs)
FOXP3 is a key transcription factor that controls the development and function of regulatory T cells (Tregs). Tregs maintain immune tolerance and prevent autoimmune disease by suppressing excessive immune responses.
FOXP3 Methylation and Autoimmunity
Methylation status of the FOXP3 gene is an important indicator of Treg activity.
In active Tregs, the FOXP3 promoter is typically hypomethylated, allowing for high levels of FOXP3 expression, which promotes the suppressive function of these cells.
Hyperactivation of the immune system!
If the immune system is highly activated--after infection or injection, Tregs are recruited to help resolve the inflammation.
FOXP3 hypomethylation is a good indicator of active immune regulation.
Hypomethylation of FOXP3 means Tregs are actively trying to control an immune response.
This could indicate a chronic inflammatory state or an attempt to prevent autoimmune reactions.
FOXP3 Hypomethylation and Autoimmune Disease
In cases where Tregs are insufficient or dysfunctional, autoimmunity can develop. If one observed FOXP3 hypomethylation, it might indicate that Tregs are being activated but are unable to fully control the immune response.
In some cases of autoimmune disease, Treg function may be impaired, leading to insufficient suppression of autoreactive immune cells.
This can be seen in conditions like autoimmune myocarditis or other autoimmune diseases like AIDP, Hashimotos, and other immune diseases.
FOXP3 hypermethylation could be a marker of reduced Treg activity, meaning that the immune system may be out of control, contributing to autoimmune disease or chronic inflammation.
Thus, FOXP3 methylation status could be used, in tandem, with time tracking.
In the early stages of an immune response, important cytokine genes (e.g., IL-6, IL-1β, TNF-α) often become hypomethylated, leading to increased expression and the release of inflammatory mediators.
These genes involved in T-cell activation and B-cell function also become hypomethylated. This allows for the differentiation of helper T cells, cytotoxic T cells, and the development of memory T cells.
CpG Islands in Immune Genes
Many immune genes contain CpG islands near their promoters. In a resting state, these regions may be methylated, keeping the genes turned off. However, during an immune response, these islands can become demethylated, activating the gene.
Tracking Hypomethylation Over Time
The timing of these changes can help track the progression of an immune response or the development of autoimmunity.
Acute response
Early hypomethylation in cytokine genes means a recent immune event.
If hypomethylation persists in certain immune regulatory genes (IL-10, FOXP3), that probably indicates an ongoing immune dysregulation or autoimmunity.
How to Measure Epigenetic Timing in Immune Responses
Epigenetic clocks for immune cells
These clocks track methylation changes over time in genes related to T-cell activation, B-cell function, and cytokine production.
By assessing the methylation patterns at various time points, researchers can estimate how long ago an immune response began.
Once again, one could lean towards sequencing.
Single-cell sequencing
Using single-cell RNA-seq or ATAC-seq combined with bisulfite sequencing might measure gene expression and methylation status at the single-cell level.
This would identify which immune cells are activated at specific times post-vaccination.
This means one might be able to track, even without knowing the time of vaccination data point exactly, when things started, to a degree.
THE HORVATH CLOCK (and others):
Horvath Clock
The Horvath clock is a type of epigenetic clock developed by Dr. Steve Horvath in 2013. It measures biological age based on the methylation patterns of specific CpG sites in the DNA, and it can predict the biological age of many tissues and organs in the body.
DNA methylation is a key part of the clock. Methyl groups attach to CpG sites (regions where a cytosine nucleotide is followed by a guanine nucleotide) and regulate gene expression. As people age, their DNA methylation patterns change in a highly predictable way at certain sites, which allows the Horvath clock to estimate the biological age of cells and tissues, often more accurately than chronological age.
The Horvath clock is based on methylation at 353 CpG sites spread across the genome. By analyzing these sites, the clock gives an estimate of biological age, which may differ from a person's chronological age.
The difference between biological and chronological age is referred to as epigenetic age acceleration. A higher biological age might suggest increased susceptibility to age-related diseases or conditions like cardiovascular disease or cancer.
A scientist might be able to tie together the Horvath clock, CpG islands, methylation states of key areas near promoters that were responsible for gene expression related to cancer, autoimmunity, and other disease states.
Immune System Clocks
The immune system clock is a concept based on the idea that the immune system’s age can be tracked through its own specific methylation patterns and cellular signatures. It focuses on immune cells and how their DNA methylation changes over time due to aging or responses to environment, like infections, or vaccines. Immune age doesn’t necessarily match chronological age. For instance, someone with a chronically activated immune system (e.g., due to infection or autoimmune disease) may have an immune system that appears "older" than their chronological age.
Epigenetic changes in immune cells, such as T-cells and B-cells, can reveal how long ago an immune response occurred and how it has evolved.
Over time, as people age or experience repeated immune system activations, the epigenetic landscape of T-cells changes, reflecting their "age" and functional state. Specific T-cell subtypes (like naive, effector, or memory T-cells) show distinct methylation profiles based on their experiences.
These are a bunch of clocks for tracking when methylation occurred:
Horvath’s Clock
epigenetic clock based on DNA methylation patterns across various tissues--can predict biological age.
Hannum's Clock
Another DNA methylation-based clock developed by Hannum et al., which focuses on blood cells. It uses a different set of CpG sites compared to Horvath’s clock--useful for estimating biological age and assessing changes in blood-related conditions and immune function.
PhenoAge Clock
Developed by Levine et al., this clock predicts biological age based on DNA methylation and is designed to be more closely related to phenotypic health-focuses on age-related health metrics, including inflammation and immune status, making it relevant for studies on immune system changes and aging.
GrimAge Clock
A DNA methylation clock developed by Lu et al. that predicts lifespan and healthspan based on epigenetic markers linked to mortality and age-related diseases--like biological age associated with cancer risk and immune dysfunction.
T-cell Senescence Clock
specifically designed to measure the epigenetic changes associated with T-cell senescence, a hallmark of immune aging--looks at T-cell function in relation to autoimmune diseases or cancer.
Cancer-Specific Methylation Clocks
These are biomarkers for cancer diagnosis and prognosis, to track epigenetic changes in tumors over time
EpiClock
integrates multiple epigenetic features to assess cellular age and function, with an emphasis on immune cells.
Here are some key genes that have CpG islands right in front of them, that can be influenced
TP53: leading to loss of tumor suppression.
CDKN2A (p16INK4a): hypermethylated in melanoma, lung cancer, and pancreatic cancer, resulting in cell cycle dysregulation.
BRCA1: Hypermethylation in breast and ovarian cancers, affecting DNA repair pathways.
VHL (Von Hippel-Lindau): Methylation--renal cell carcinoma by inactivating this tumor suppressor gene.
MGMT: Hypermethylation glioblastoma
PTEN: prostate cancer.
MLH1: Lynch syndrome-related cancers
MBD4: many cancers
SFRP1: colorectal cancer, affecting the Wnt signaling pathway.
RASSF1A: lung cancer and other malignancies, impacting cell cycle regulation and apoptosis.
DAPK (Death-Associated Protein Kinase): Hypermethylation can lead to loss of apoptotic regulation in several cancers.
ER (Estrogen Receptor): breast cancer can result in altered hormone signaling.
PR (Progesterone Receptor): Similar to ER, in breast cancer.
CTLA-4: lupus and rheumatoid arthritis.
FOXP3: autoimmune disorders.
BDNF (Brain-Derived Neurotrophic Factor): various neurological conditions, including depression and schizophrenia.
APC (Adenomatous Polyposis Coli): Hypermethylation linked to colorectal cancer, involved in Wnt signaling.
STK11 (LKB1): Hypermethylation is associated with Peutz-Jeghers syndrome and lung cancer.
TGFBR2 (Transforming Growth Factor Beta Receptor 2): Hypermethylation is common in colorectal cancer and is associated with TGF-β signaling disruption.
NDRG1: Involved in cancer metastasis, hypermethylation can lead to decreased expression in various cancers.
SOCS1 (Suppressor of Cytokine Signaling 1): Hypermethylation linked to several hematological malignancies, affecting immune response.
DAPK1: Associated with the apoptotic process, hypermethylation is observed in various cancers.
Genes Involved in DNA Repair and Genomic Stability
XPF: Hypermethylation can affect DNA repair processes in cancers.
FANCF: Associated with Fanconi anemia, hypermethylation may lead to increased cancer susceptibility.
BRCA2: Involved in DNA repair; hypermethylation may affect breast and ovarian cancer susceptibility.
Genes in Development and Differentiation
HIC1 (Hypermethylated in Cancer 1): Hypermethylation in various cancers; involved in transcriptional repression.
GATA5: Hypermethylation linked to colorectal cancer, influencing differentiation processes.
SALL3: A transcription factor that can be hypermethylated in Wilms tumor and other cancers.
Additional Cancer-Related Genes
PAX5: Hypermethylation is associated with B-cell malignancies.
RARB (Retinoic Acid Receptor Beta): Linked to breast cancer
FHIT (Fragile Histidine Triad): lung cancer and other malignancies.
NDRG2: expression in tumors.
PRDM1 (Blimp-1): lymphomas and immune responses.
AR (Androgen Receptor): prostate cancer
CDH1 (E-cadherin): epithelial-to-mesenchymal transition in cancers.
IL-2RA (CD25): autoimmune diseases like multiple sclerosis.
Neurological and Psychiatric Disorder Genes
NRG1 (Neuregulin 1): schizophrenia.
SLC6A4 (Serotonin Transporter): depression susceptibility.
MECP2: neurodevelopmental disorders.
MMP2 (Matrix Metalloproteinase 2): cancers affecting tissue remodeling.
TET2: blood cancers like AML (Acute Myeloid Leukemia).
But how could this occur without integration?
transient exposure to linearized DNA within lipid nanoparticles, can cause transient expression AND cause HYPERMETHYLATION of the CpG islands associated with specific promoter regions in our genes!
Once again, CpG islands are regions rich in CG dinucleotides, typically located near gene promoters, and their methylation status often regulates gene expression
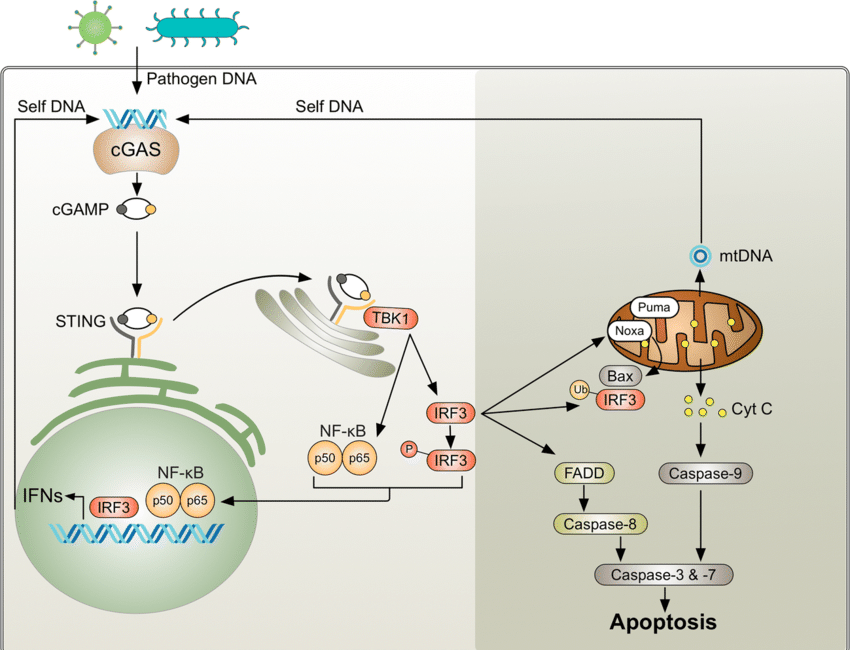
cGAS STING!
Linearized plasmid DNA can induce CpG island hypermethylation through cellular stress and immune responses triggered by foreign DNA recognition. When introduced into cells, linearized DNA activates DNA-sensing pathways, like cGAS-STING pathway, which can initiate downstream epigenetic changes, including methylation of CpG islands at gene promoters. In response to perceived DNA damage or foreign DNA presence, DNA methyltransferases (DNMTs) may be recruited to CpG-rich promoter regions, catalyzing hypermethylation, leading to chromatin condensation, preventing the binding of transcription factors and silencing gene expression. Additionally, immune responses induced by exogenous DNA may activate inflammatory signaling, which can epigenetically modify host genome regions, particularly CpG islands in promoters of genes involved in immune regulation or DNA repair (CANCER)
Ultimately, hypermethylation of CpG islands alters promoter activity by repressing transcriptional initiation, effectively silencing or downregulating genes involved in stress responses, immunity, or cell cycle regulation.
When cGAS-STING is activated by plasmid DNA, it triggers an immune response, leading to the recruitment of DNA methyltransferases (DNMTs) to CpG islands at gene promoters. This causes hypermethylation, which silences gene expression by blocking transcription factor binding and promoting chromatin condensation
( This proposal needs correction by those who may perform in a lab and probable edits. )
So now we look at another study:
Plasmid DNA is a contaminant in 💉🦠🧬and is double stranded (ds) DNA: it contains CpG oligonucleotide (ODN).
A STUDY: Researchers found differences in cellular responses+ different gene responses to ds DNA and its effect on GENE MODIFICATION--transient transfection.
The STUDY "Differential cellular responses to exogenous DNA in mammalian cells and its effect on oligonucleotide directed gene modification" Igoucheva, O et al. Gene therapy vol. 13,3 (2006): 266-75. doi:10.1038/sj.gt.3302643 https://sci-hub.se/10.1038/sj.gt.3302643
(this is from a twitter thread I did).
Researchers wanted to know how cells respond to the presence of double-stranded DNA (dsDNA), and the influence that different dsDNA (different sizes/types) have on different cellular processes, and the impact on different genes. They found a variety of results.
They used two types of cell lines, NIH3T3 and CHO-K1, and introduced plasmid dsDNA into the cells. The introduction of dsDNA was achieved by transient transfection, where external genetic material (in this case, dsDNA) is introduced into cells (DNA/lipid complex).
After adding the dsDNA to the cells, the researchers observed how the cells' gene expression changed, especially the transcriptional response, which means they studied how genes were "turned on" or "turned off" in response to the presence of dsDNA inside the cells.
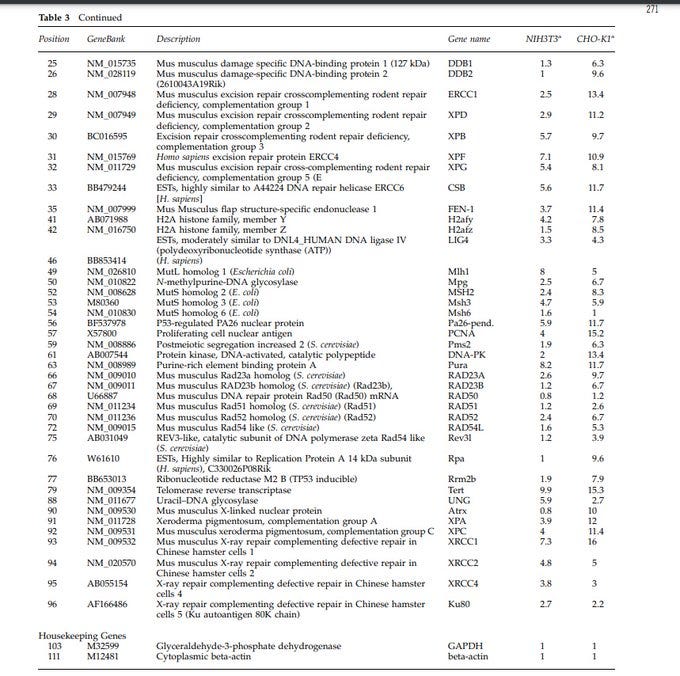
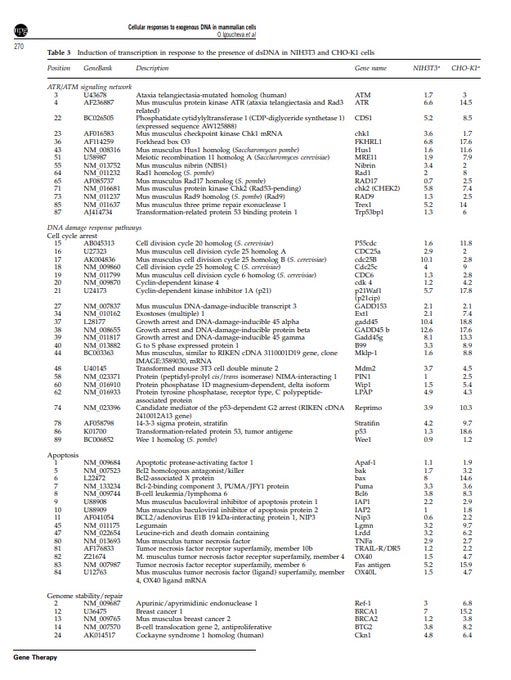
The researchers analyzed the activity of various genes in response to dsDNA. The genes they examined are associated with processes like DNA repair, cell cycle regulation, apoptosis (cell death), and other cellular responses, such as uncontrolled cell growth, and cancer.
They found "cell-type dependency". Different cell types exhibit remarkably different rates of gene modification in response to the dsDNA.
Introduction of dsDNA activated transcription of many genes involved in DNA damage signaling and repair.
Long dsDNA induced genes responsible for sensing DNA damage, like ATR-dependent signaling, nucleotide excision repair (NER), and mismatch repair (MMR).
ATR (ataxia telangiectasia and Rad3-related) was identified as the primary sensor of DNA replication blockage resulting from lesions by DNA adducts, UV, and DNA synthesis inhibitors.
The study observed a strong induction of ATR and several genes participating in ATR-dependent signaling.
ATR is paramount in maintaining stability of the genome.
Mutations or dysregulation of ATR can lead to disease and cancer.
ATR is also part of the activation of cell cycle checkpoints. Dysregulation is another hallmark in cancer. ATR is also a tumor suppressor. Loss of function or mutations in ATR may contribute to the development of cancer.
This table shows transcription in response to the presence of dsDNA in the two cell types. The data is for various genes associated w/ different cellular pathways. The fold induction values represent ratio of intensity of each gene in cells transfected.
The ones that are related to cancer: ATR (Ataxia Telangiectasia and Rad3 Related): Implication in Cancer: Dysregulation of ATR has been associated with genomic instability and cancer. ATR mutations or altered expression can contribute to uncontrolled cell proliferation.
ATM (Ataxia Telangiectasia Mutated): ATM is involved in DNA repair and cell cycle control. Mutations in ATM are linked to an increased risk of cancer. ATM is a tumor suppressor, and its dysfunction can lead to genomic instability and cancer development.
Cyclin-Dependent Kinase Inhibitor 1A (p21): p21 is a cyclin-dependent kinase inhibitor, regulating the cell cycle. p21 acts as a tumor suppressor by inhibiting cell cycle progression. Dysregulation can lead to uncontrolled cell division and contribute to cancer.
Bcl-2 Homologous Antagonist/Killer (Bak): apoptosis and regulation of cell death. Dysregulation of apoptosis is a common feature in cancer. Altered Bak function may affect the balance between cell survival and death, contributing to cancer development.
B-cell Leukemia/Lymphoma 6 (Bcl6): cell cycle regulation and apoptosis. Aberrant expression of Bcl6 is associated with lymphomas and other cancers. It can promote cell survival and inhibit apoptosis, contributing to tumor development.
Growth Arrest and DNA-Damage-Inducible 45 (GADD45): cell cycle arrest and DNA damage response. GADD45 genes play a role in preventing genomic instability. Dysregulation can contribute to cancer by affecting cell cycle control and DNA repair.
p53 (Tumor Protein 53): tumor suppressor, regulating the cell cycle, DNA repair, and apoptosis. Mutations in p53 are common in various cancers. Loss of p53 function allows for uncontrolled cell division and survival of damaged cells, contributing to cancer progression.
BRCA1 and BRCA2: DNA repair.
Mutations in BRCA1 and BRCA2 are associated with an increased risk of breast and ovarian cancers. genomic integrity. *** Tumor Necrosis Factor (TNF): apoptosis and inflammation. Dysregulation of TNF signaling : chronic inflammation and cancer.
Telomerase Reverse Transcriptase (TERT): maintains telomere length. Activation of telomerase, including TERT, is common in cancer cells, allowing for unlimited cell division. unlimited cell division
Xeroderma Pigmentosum Genes (XPA, XPC): DNA repair. I Mutations in XPA and XPC are associated with an increased susceptibility to skin cancer. These genes play a crucial role in repairing DNA damage caused by UV radiation.
Nuclear Protein and Cellular Processes— nuclear protein (PA26) associated with the gene suggests involvement in nuclear activities (nucleus). (BF537978) may be under the regulation of the P53 protein. Both were impacted in this study by the dsDNA, including other genes.
other studies exist on the impact of dsDNA on signaling pathways, and specific genes.
cGAS STING and APOBEC:
cGAS STING is part of this, and so are APOBEC enzymes (and macrophages)
For a refresher and a very long read on cGAS STING activation without any kind of integration happening, read this long novel:
What we are look9ing at, is combining the cGAS STING activation pathway, which can occur with DNA plasmids (longer ones even in smaller amounts will drive the highest activation intensity (and spike, double stranded RNA, double stranded DNA, bacteria, and some charged elements too), that gets activated, then this pathway is involved with APOBEC enzymes.
DNA Plasmid Detection by cGAS
Cytosolic DNA from foreign sources (like DNA plasmids, viral DNA, or self-DNA from damaged mitochondria or nuclei) is usually considered abnormal by the immune system.
cGAS (cyclic GMP-AMP synthase) is a cytosolic DNA sensor that detects these foreign or mislocated DNA fragments.
When DNA plasmids enter the cytoplasm, cGAS recognizes double-stranded DNA (dsDNA) of a particular length (usually >40 base pairs) and becomes activated.
Upon activation, cGAS synthesizes a second messenger called cyclic GMP-AMP (cGAMP) from ATP and GTP. This cGAMP molecule is a key activator of the STING protein.
STING Activation and Immune Signaling
STING (Stimulator of Interferon Genes) resides on the endoplasmic reticulum (ER) and gets activated by the cGAMP produced by cGAS.
Once STING is activated, it translocates from the ER to the Golgi apparatus, where it serves as a platform to recruit and activate other key signaling molecules like:
TBK1 (TANK-binding kinase 1)
IRF3 (Interferon Regulatory Factor 3)
STING also activates NF-KB, a transcription factor that promotes the expression of proinflammatory cytokines like TNF-a, IL-6, and type I interferons
This inflammatory response can be beneficial in clearing infections, but when chronically activated (as with persistent DNA plasmid exposure), it can lead to tissue damage, immune dysregulation, and promote disease.
APOBEC Activation and DNA Editing
APOBEC (Apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like) family of cytidine deaminases is part of the immune system's response to foreign DNA.
APOBEC enzymes are usually upregulated in response to viral infections and inflammatory signals (such as those triggered by the cGAS-STING pathway).
These enzymes deaminate cytosines in single-stranded DNA (ssDNA), converting cytosine (C) to uracil (U). This can lead to C→T or C→G mutations during DNA replication.
APOBEC3A and APOBEC3B, in particular, have been implicated in cancer due to their mutagenic potential.
They preferentially target single-stranded DNA, which is often transiently exposed during DNA replication, transcription, or repair.
Mutations caused by APOBEC enzymes often leave a characteristic mutational signature, known as the APOBEC signature, in the genome, contributing to genomic instability.
Chronic Inflammation, ROS, and Epigenetic Changes
Persistent activation of STING induces a chronic inflammatory environment with high levels of cytokines and reactive oxygen species (ROS).
ROS causes oxidative damage to DNA and proteins, leading to mutations and further instability.
Chronic inflammation also disrupts the normal activity of epigenetic regulators, especially DNA methyltransferases (DNMTs).
DNMT1 maintains existing DNA methylation patterns, while DNMT3A and DNMT3B are responsible for de novo methylation (adding methyl groups to previously unmethylated DNA).
Inflammation-induced ROS and cytokines, combined with cGAS-STING pathway activity, can dysregulate these DNMTs, leading to aberrant DNA methylation.
CpG Island Hypermethylation
CpG islands are regions of DNA rich in cytosine (C) and guanine (G) dinucleotides. They are commonly found in the promoter regions of genes, especially tumor suppressor genes and other regulatory elements.
DNA methylation at CpG islands involves adding a methyl group (-CH3) to the cytosine residue, typically silencing gene expression.
In the context of chronic inflammation and cGAS-STING activation, DNMTs can become overactive or improperly regulated, leading to hypermethylation of CpG islands.
Hypermethylation of promoter CpG islands can silence tumor suppressor genes such as p53, RB1, BRCA1, CDKN2A, or PTEN.
This gene silencing prevents these proteins from performing their normal roles in controlling the cell cycle, promoting apoptosis (programmed cell death), and repairing damaged DNA.
APOBEC-Induced Mutations and Cancer
APOBEC enzymes, particularly APOBEC3B, are often upregulated in cancers and can cause C-to-T or C-to-G mutations.
These mutations accumulate in the genome, promoting genomic instability. This, coupled with the silencing of tumor suppressor genes through CpG island hypermethylation, creates an environment conducive to tumorigenesis.
Cancer types where the APOBEC mutational signature is prevalent include breast cancer, lung cancer, bladder cancer, and others.
Autoimmune Disease Mechanism
Chronic cGAS-STING pathway activation can lead to autoimmune diseases by promoting the constant production of type I interferons (IFN-a, IFN-b) and proinflammatory cytokines (IL-6, TNF-a).
This hyperactive immune response can lead to autoimmunity because the body begins attacking its own cells and tissues.
SLE (systemic lupus erythematosus) is a classic autoimmune disease associated with cGAS-STING overactivation. In lupus, there is excessive recognition of self-DNA, driving a hyperinflammatory state.
APOBEC-induced mutations can also alter the antigenic profile of cells, which may promote autoimmune reactions as the immune system mistakenly identifies altered self-cells as foreign.
Macrophage Activation and Inflammatory Disease
Macrophages are immune cells that respond strongly to cGAS-STING activation. When activated by STING, macrophages produce high levels of proinflammatory cytokines like TNF-a, IL-6, and IL-1b.
Chronic macrophage activation can lead to tissue damage and contribute to autoimmune diseases or hyperinflammatory syndromes, such as macrophage activation syndrome (MAS).
In diseases like Adult-onset Still’s disease (AOSD), macrophage hyperactivation driven by the cGAS-STING pathway results in widespread inflammation.
Overactive macrophages in the context of chronic inflammation can perpetuate disease progression by continuously driving inflammatory signals and promoting tissue destruction.
Disease Outcomes
Cancer: The combined effects of APOBEC-induced mutagenesis and CpG island hypermethylation promote cancer development by driving genomic instability, silencing tumor suppressor genes, and activating oncogenes.
Autoimmune Diseases: Chronic activation of the cGAS-STING pathway leads to autoimmune disorders like SLE by generating self-DNA recognition, continuous type I interferon production, and inappropriate immune responses.
Hyperinflammatory Syndromes: Macrophage hyperactivation, resulting from STING signaling, can lead to hyperinflammatory conditions like MAS, AOSD, and other autoimmune or inflammatory diseases.
Summary of the Detailed Pathway:
DNA plasmids enter the cytoplasm, activating cGAS.
cGAS produces cGAMP, activating STING.
STING triggers immune signaling through TBK1, NF-kB, and IRF3, leading to the production of cytokines and interferons.
APOBEC enzymes are activated and induce cytosine deamination, causing C→T or C→G mutations in DNA.
Chronic inflammation and ROS production disrupt DNMT function, leading to hypermethylation of CpG islands.
Hypermethylation silences tumor suppressor genes, while APOBEC mutations contribute to genomic instability, driving cancer.
Chronic STING activation drives autoimmune diseases through continuous type I interferon production.
Macrophage hyperactivation results in hyperinflammatory syndromes like MAS and AOSD.
If we pull this into a short highlighted cliff notes section:
DNA plasmids enter the cytoplasm, activating cGAS.
cGAS produces cGAMP, activating STING.
STING triggers immune signaling through TBK1, NF-κB, and IRF3, leading to the production of cytokines and interferons.
APOBEC enzymes are activated and induce cytosine deamination, causing C→T or C→G mutations in DNA.
Chronic inflammation and ROS production disrupt DNMT function, leading to hypermethylation of CpG islands.
Hypermethylation silences tumor suppressor genes, while APOBEC mutations contribute to genomic instability, driving cancer. This can also drive autoimmune states.
Chronic STING activation drives autoimmune diseases through continuous type I interferon production.
Macrophage hyperactivation results in hyperinflammatory syndromes like MAS and AOSD
These tests could be used to quantify any type of medication injury, any drug injury, not just things with modified RNA in them, or DNA plasmids, or spike protein. You could use a test system like this (of course there is more lab work demanded—this is just highlights, which feels wild to say, considering the length of this, you could run these tests on someone who was injured by attenuated vaccines. You could run these types of tests checking methylation status on those who got Remdesvir—the sky is really the limit on running this type of test.
The first study mentioned—it was tracked that those with a significant immune system response lost a couple of years of their life. YEARS.
What happens to those with injuries? What happens to those who had serious infections AND injuries?
These are the questions that need to be answered.
Sources:
pmc.ncbi.nlm.nih.gov/articles/PMC9203887/… https://nature.com/articles/1205600… https://pubmed.ncbi.nlm.nih.gov/33979432/ https://pmc.ncbi.nlm.nih.gov/articles/PMC10487967/



Contribution of the cGAS-STING to the progressive degeneration of amyotrophic lateral sclerosis (ALS) / Lou Gehrig's disease
cGAS and DDX41-STING mediated intrinsic immunity spreads intercellularly to promote neuroinflammation in SOD1 ALS model (2022)
SUMMARY
Neuroinflammation exacerbates the progression of SOD1-driven amyotrophic lateral sclerosis (ALS), although the underlying mechanisms remain largely unknown. Herein, we demonstrate that misfolded SOD1 (SOD1Mut)-causing ALS results in mitochondrial damage, thus triggering the release of mtDNA and an RNA:DNA hybrid into the cytosol in an mPTP-independent manner to activate IRF3- and IFNAR-dependent type I interferon (IFN-I) and interferon-stimulating genes. The neuronal hyper-IFN-I and pro-inflammatory responses triggered in ALS-SOD1Mut were sufficiently robust to cause a strong physiological outcome in vitro and in vivo. cGAS/DDX41-STING-signaling is amplified in bystander cells through inter-neuronal gap junctions. Our results highlight the importance of a common DNA-sensing pathway between SOD1 and TDP-43 in influencing the progression of ALS.
https://pmc.ncbi.nlm.nih.gov/articles/PMC9194172/
Thanks Christie.
When you mentioned CpG islands G4Ps sprung to mind, as these are also associated with C & G, as well as mRNA m1u gene therapies:
DNA G-quadruplex stability, position and chromatin accessibility are associated with CpG island methylation (2020)
"... Using methylation data from human embryonic stem cells (hESCs) and three hESC-derived populations, we showed that hypomethylated CpGs located inside CGI (CGI/CpG) tend to be associated with highly stable G4FS (Minimum free energy ≤ -30 kcal·mol-1 )."
https://pubmed.ncbi.nlm.nih.gov/31532882/